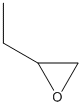
1,2-epoxybutane
General
Type : Epoxide
Chemical_Nomenclature : 2-ethyloxirane
Canonical SMILES : CCC1CO1
InChI : InChI=1S\/C4H8O\/c1-2-4-3-5-4\/h4H,2-3H2,1H3
InChIKey : RBACIKXCRWGCBB-UHFFFAOYSA-N
Other name(s) : 1,2-Butylene oxide, Ethyloxirane, CHEBI:82326, CHEMBL1378095
Target
Families : Epoxide_hydrolase
References (4)
| Title : An epoxide hydrolase from endophytic Streptomyces shows unique structural features and wide biocatalytic activity - Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| Author(s) : Tormet-Gonzalez GD , Wilson C , de Oliveira GS , dos Santos JC , de Oliveira LG , Dias MVB |
| Ref : Acta Crystallographica D Struct Biol , 76 :868 , 2020 |
| Abstract : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| ESTHER : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| PubMedSearch : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| PubMedID: 32876062 |
| Gene_locus related to this paper: strsq-b1eph2 |
| Title : Cloning and characterization of an epoxide hydrolase from Novosphingobium aromaticivorans - Woo_2009_Appl.Microbiol.Biotechnol_82_873 |
| Author(s) : Woo JH , Kang JH , Kang SG , Hwang YO , Kim SJ |
| Ref : Applied Microbiology & Biotechnology , 82 :873 , 2009 |
| Abstract : Woo_2009_Appl.Microbiol.Biotechnol_82_873 |
| ESTHER : Woo_2009_Appl.Microbiol.Biotechnol_82_873 |
| PubMedSearch : Woo_2009_Appl.Microbiol.Biotechnol_82_873 |
| PubMedID: 19083233 |
| Gene_locus related to this paper: novad-q2g620 |
| Title : Biochemical characterization and transcriptional analysis of the epoxide hydrolase from white-rot fungus Phanerochaete chrysosporium - Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| Author(s) : Li N , Zhang Y , Feng H |
| Ref : Acta Biochim Biophys Sin (Shanghai) , 41 :638 , 2009 |
| Abstract : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| ESTHER : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| PubMedSearch : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| PubMedID: 19657565 |
| Gene_locus related to this paper: phach-b0fsq0 |
| Title : A cold-adapted epoxide hydrolase from a strict marine bacterium, Sphingophyxis alaskensis - Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| Author(s) : Kang JH , Woo JH , Kang SG , Hwang YO , Kim SJ |
| Ref : J Microbiol Biotechnol , 18 :1445 , 2008 |
| Abstract : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| ESTHER : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedSearch : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedID: 18756107 |
| Gene_locus related to this paper: 9sphn-q3vax0 |