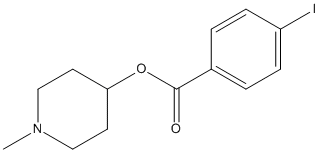
[I125]Methyl-4-piperidinyl-N-benzoate
General
Type : PET probe || Benzoate
Chemical_Nomenclature : (1-methylpiperidin-4-yl) 4-iodobenzoate
Canonical SMILES : CN1CCC(CC1)OC(=O)C2=CC=C(C=C2)I
InChI : InChI=1S\/C13H16INO2\/c1-15-8-6-12(7-9-15)17-13(16)10-2-4-11(14)5-3-10\/h2-5,12H,6-9H2,1H3
InChIKey : KRVXRNGJVZIXHS-UHFFFAOYSA-N
Other name(s) : 1-Methylpiperidin-4-yl 4-iodobenzoate, [I125]MP4Bz, CHEMBL83382, SCHEMBL1839004, BDBM50059308
Target
Families : BCHE
References (3)
| Title : Butyrylcholinesterase radioligands to image Alzheimer's disease brain - Darvesh_2013_Chem.Biol.Interact_203_354 |
| Author(s) : Darvesh S |
| Ref : Chemico-Biological Interactions , 203 :354 , 2013 |
| Abstract : Darvesh_2013_Chem.Biol.Interact_203_354 |
| ESTHER : Darvesh_2013_Chem.Biol.Interact_203_354 |
| PubMedSearch : Darvesh_2013_Chem.Biol.Interact_203_354 |
| PubMedID: 22935510 |
| Title : Synthesis and preliminary evaluation of piperidinyl and pyrrolidinyl iodobenzoates as imaging agents for butyrylcholinesterase - Macdonald_2011_Mol.Imaging.Biol_13_1250 |
| Author(s) : Macdonald IR , Reid GA , Joy EE , Pottie IR , Matte G , Burrell S , Mawko G , Martin E , Darvesh S |
| Ref : Mol Imaging Biol , 13 :1250 , 2011 |
| Abstract : Macdonald_2011_Mol.Imaging.Biol_13_1250 |
| ESTHER : Macdonald_2011_Mol.Imaging.Biol_13_1250 |
| PubMedSearch : Macdonald_2011_Mol.Imaging.Biol_13_1250 |
| PubMedID: 20976626 |
| Title : Synthesis of carbon-11- and fluorine-18-labeled 1-methyl-4-piperidyl-4'-fluorobenzoate and their biodistribution in mice - Bormans_1996_Nucl.Med.Biol_23_513 |
| Author(s) : Bormans G , Sherman P , Snyder SE , Kilbourn MR |
| Ref : Nucl Med Biol , 23 :513 , 1996 |
| Abstract : Bormans_1996_Nucl.Med.Biol_23_513 |
| ESTHER : Bormans_1996_Nucl.Med.Biol_23_513 |
| PubMedSearch : Bormans_1996_Nucl.Med.Biol_23_513 |
| PubMedID: 8832709 |