[18F]FEtP4A
General
Type : Piperidine || PET probe
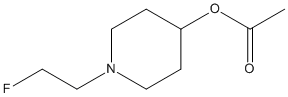
Chemical_Nomenclature : [1-(2-fluoroethyl)piperidin-4-yl] acetate, [1-(2-(18F)fluoranylethyl)piperidin-4-yl] acetate
Canonical SMILES : CC(=O)OC1CCN(CC1)CCF
InChI : InChI=1S\/C9H16FNO2\/c1-8(12)13-9-2-5-11(6-3-9)7-4-10\/h9H,2-7H2,1H3 || InChI=1S\/C9H16FNO2\/c1-8(12)13-9-2-5-11(6-3-9)7-4-10\/h9H,2-7H2,1H3\/i10-1
InChIKey : LGIBFTXNELWMLC-UHFFFAOYSA-N || LGIBFTXNELWMLC-LMANFOLPSA-N
Other name(s) : CHEMBL427537, N-[18F]fluoroethyl-4-piperidyl acetate, MOLI000426, CHEMBL1169458
MW : 189.23
Formula : C9H16FNO2
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : No family
References (3)
| Title : N-[18F] fluoroethylpiperidin-4ylmethyl acetate, a novel lipophilic acetylcholine analogue for PET measurement of brain acetylcholinesterase activity - Kikuchi_2005_J.Med.Chem_48_2577 |
| Author(s) : Kikuchi T , Zhang MR , Ikota N , Fukushi K , Okamura T , Suzuki K , Arano Y , Irie T |
| Ref : Journal of Medicinal Chemistry , 48 :2577 , 2005 |
| Abstract : Kikuchi_2005_J.Med.Chem_48_2577 |
| ESTHER : Kikuchi_2005_J.Med.Chem_48_2577 |
| PubMedSearch : Kikuchi_2005_J.Med.Chem_48_2577 |
| PubMedID: 15801847 |
| Title : N-[18F]fluoroethyl-4-piperidyl acetate ([18F]FEtP4A): A PET tracer for imaging brain acetylcholinesterase in vivo - Zhang_2003_Bioorg.Med.Chem_11_2519 |
| Author(s) : Zhang MR , Furutsuka K , Maeda J , Kikuchi T , Kida T , Okauchi T , Irie T , Suzuki K |
| Ref : Bioorganic & Medicinal Chemistry , 11 :2519 , 2003 |
| Abstract : Zhang_2003_Bioorg.Med.Chem_11_2519 |
| ESTHER : Zhang_2003_Bioorg.Med.Chem_11_2519 |
| PubMedSearch : Zhang_2003_Bioorg.Med.Chem_11_2519 |
| PubMedID: 12757720 |
| Title : Synthesis and preliminary evaluation of [18F]FEtP4A, a promising PET tracer for mapping acetylcholinesterase in vivo - Zhang_2002_Nucl.Med.Biol_29_463 |
| Author(s) : Zhang MR , Tsuchiyama A , Haradahira T , Furutsuka K , Yoshida Y , Kida T , Noguchi J , Irie T , Suzuki K |
| Ref : Nucl Med Biol , 29 :463 , 2002 |
| Abstract : Zhang_2002_Nucl.Med.Biol_29_463 |
| ESTHER : Zhang_2002_Nucl.Med.Biol_29_463 |
| PubMedSearch : Zhang_2002_Nucl.Med.Biol_29_463 |
| PubMedID: 12031881 |