[11C]-MP4A
General
Type : Acetate || Piperidine || PET probe
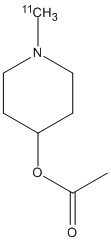
Chemical_Nomenclature : (1-methylpiperidin-4-yl) acetate
Canonical SMILES : CC(=O)OC1CCN(CC1)C
InChI : InChI=1S\/C8H15NO2\/c1-7(10)11-8-3-5-9(2)6-4-8\/h8H,3-6H2,1-2H3\/i2-1
InChIKey : RWPYOAUYOSFJQV-JVVVGQRLSA-N
Other name(s) : [11C]MP4A, [11C]AMP, 11C-MP4A, 1-[11C]methylpiperidin acetate, N-[11C]methylpiperidinyl-4-acetate, (1-methylpiperidin-4-yl) acetate, AC1L9OVG, CHEMBL2311149, MOLI000787, MOLI001520
MW : 156.21
Formula : C8H14N
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : ACHE
References (26)
| Title : PET Imaging of Cholinergic Neurotransmission in Neurodegenerative Disorders - Tiepolt_2022_J.Nucl.Med_63_33S |
| Author(s) : Tiepolt S , Meyer PM , Patt M , Deuther-Conrad W , Hesse S , Barthel H , Sabri O |
| Ref : J Nucl Med , 63 :33S , 2022 |
| Abstract : Tiepolt_2022_J.Nucl.Med_63_33S |
| ESTHER : Tiepolt_2022_J.Nucl.Med_63_33S |
| PubMedSearch : Tiepolt_2022_J.Nucl.Med_63_33S |
| PubMedID: 35649648 |
| Title : Voxel-Based Acetylcholinesterase PET Study in Early and Late Onset Alzheimer's Disease - Hirano_2018_J.Alzheimers.Dis_62_1539 |
| Author(s) : Hirano S , Shinotoh H , Shimada H , Ota T , Sato K , Tanaka N , Zhang MR , Higuchi M , Fukushi K , Irie T , Kuwabara S , Suhara T |
| Ref : J Alzheimers Dis , 62 :1539 , 2018 |
| Abstract : Hirano_2018_J.Alzheimers.Dis_62_1539 |
| ESTHER : Hirano_2018_J.Alzheimers.Dis_62_1539 |
| PubMedSearch : Hirano_2018_J.Alzheimers.Dis_62_1539 |
| PubMedID: 29562505 |
| Title : PET probes for imaging brain acetylcholinesterase - Kikuchi_2013_J.Labelled.Comp.Radiopharm_56_172 |
| Author(s) : Kikuchi T , Okamura T , Zhang MR , Irie T |
| Ref : J Labelled Comp Radiopharm , 56 :172 , 2013 |
| Abstract : Kikuchi_2013_J.Labelled.Comp.Radiopharm_56_172 |
| ESTHER : Kikuchi_2013_J.Labelled.Comp.Radiopharm_56_172 |
| PubMedSearch : Kikuchi_2013_J.Labelled.Comp.Radiopharm_56_172 |
| PubMedID: 24285323 |
| Title : Cholinergic system function and cognition in mild cognitive impairment - Haense_2012_Neurobiol.Aging_33_867 |
| Author(s) : Haense C , Kalbe E , Herholz K , Hohmann C , Neumaier B , Krais R , Heiss WD |
| Ref : Neurobiology of Aging , 33 :867 , 2012 |
| Abstract : Haense_2012_Neurobiol.Aging_33_867 |
| ESTHER : Haense_2012_Neurobiol.Aging_33_867 |
| PubMedSearch : Haense_2012_Neurobiol.Aging_33_867 |
| PubMedID: 20961662 |
| Title : Reduced acetylcholinesterase activity in the fusiform gyrus in adults with autism spectrum disorders - Suzuki_2011_Arch.Gen.Psychiatry_68_306 |
| Author(s) : Suzuki K , Sugihara G , Ouchi Y , Nakamura K , Tsujii M , Futatsubashi M , Iwata Y , Tsuchiya KJ , Matsumoto K , Takebayashi K , Wakuda T , Yoshihara Y , Suda S , Kikuchi M , Takei N , Sugiyama T , Irie T , Mori N |
| Ref : Arch Gen Psychiatry , 68 :306 , 2011 |
| Abstract : Suzuki_2011_Arch.Gen.Psychiatry_68_306 |
| ESTHER : Suzuki_2011_Arch.Gen.Psychiatry_68_306 |
| PubMedSearch : Suzuki_2011_Arch.Gen.Psychiatry_68_306 |
| PubMedID: 21383265 |
| Title : Use of a novel radiometric method to assess the inhibitory effect of donepezil on acetylcholinesterase activity in minimally diluted tissue samples - Kikuchi_2010_Br.J.Pharmacol_159_1732 |
| Author(s) : Kikuchi T , Okamura T , Arai T , Obata T , Fukushi K , Irie T , Shiraishi T |
| Ref : British Journal of Pharmacology , 159 :1732 , 2010 |
| Abstract : Kikuchi_2010_Br.J.Pharmacol_159_1732 |
| ESTHER : Kikuchi_2010_Br.J.Pharmacol_159_1732 |
| PubMedSearch : Kikuchi_2010_Br.J.Pharmacol_159_1732 |
| PubMedID: 20401964 |
| Title : Development of Alzheimer's disease imaging agents for clinical studies - Ryu_2008_Front.Biosci_13_777 |
| Author(s) : Ryu EK , Chen X |
| Ref : Front Biosci , 13 :777 , 2008 |
| Abstract : Ryu_2008_Front.Biosci_13_777 |
| ESTHER : Ryu_2008_Front.Biosci_13_777 |
| PubMedSearch : Ryu_2008_Front.Biosci_13_777 |
| PubMedID: 17981587 |
| Title : Cerebral acetylcholinesterase imaging: development of the radioprobes - Kikuchi_2007_Curr.Top.Med.Chem_7_1790 |
| Author(s) : Kikuchi T , Okamura T , Fukushi K , Takahashi K , Toyohara J , Okada M , Zhang MR , Irie T |
| Ref : Curr Top Med Chem , 7 :1790 , 2007 |
| Abstract : Kikuchi_2007_Curr.Top.Med.Chem_7_1790 |
| ESTHER : Kikuchi_2007_Curr.Top.Med.Chem_7_1790 |
| PubMedSearch : Kikuchi_2007_Curr.Top.Med.Chem_7_1790 |
| PubMedID: 17979787 |
| Title : [Imaging of brain acetylcholinesterase activity in dementias and extrapyramidal disorders] - Shinotoh_2007_Rinsho.Shinkeigaku_47_822 |
| Author(s) : Shinotoh H |
| Ref : Rinsho Shinkeigaku , 47 :822 , 2007 |
| Abstract : Shinotoh_2007_Rinsho.Shinkeigaku_47_822 |
| ESTHER : Shinotoh_2007_Rinsho.Shinkeigaku_47_822 |
| PubMedSearch : Shinotoh_2007_Rinsho.Shinkeigaku_47_822 |
| PubMedID: 18210807 |
| Title : Cortical acetylcholine esterase activity and ApoE4-genotype in Alzheimer disease - Eggers_2006_Neurosci.Lett_408_46 |
| Author(s) : Eggers C , Herholz K , Kalbe E , Heiss WD |
| Ref : Neuroscience Letters , 408 :46 , 2006 |
| Abstract : Eggers_2006_Neurosci.Lett_408_46 |
| ESTHER : Eggers_2006_Neurosci.Lett_408_46 |
| PubMedSearch : Eggers_2006_Neurosci.Lett_408_46 |
| PubMedID: 16996687 |
| Title : Estimation of plasma IC50 of donepezil hydrochloride for brain acetylcholinesterase inhibition in monkey using N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and PET - Shiraishi_2005_Neuropsychopharmacology_30_2154 |
| Author(s) : Shiraishi T , Kikuchi T , Fukushi K , Shinotoh H , Nagatsuka S , Tanaka N , Ota T , Sato K , Hirano S , Tanada S , Iyo M , Irie T |
| Ref : Neuropsychopharmacology , 30 :2154 , 2005 |
| Abstract : Shiraishi_2005_Neuropsychopharmacology_30_2154 |
| ESTHER : Shiraishi_2005_Neuropsychopharmacology_30_2154 |
| PubMedSearch : Shiraishi_2005_Neuropsychopharmacology_30_2154 |
| PubMedID: 15920507 |
| Title : Acetylcholinesterase imaging: its use in therapy evaluation and drug design - Shinotoh_2004_Curr.Pharm.Des_10_1505 |
| Author(s) : Shinotoh H , Fukushi K , Nagatsuka S , Irie T |
| Ref : Curr Pharm Des , 10 :1505 , 2004 |
| Abstract : Shinotoh_2004_Curr.Pharm.Des_10_1505 |
| ESTHER : Shinotoh_2004_Curr.Pharm.Des_10_1505 |
| PubMedSearch : Shinotoh_2004_Curr.Pharm.Des_10_1505 |
| PubMedID: 15134572 |
| Title : Effects of acute acetylcholinesterase inhibition on the cerebral cholinergic neuronal system and cognitive function: Functional imaging of the conscious monkey brain using animal PET in combination with microdialysis - Tsukada_2004_Synapse_52_1 |
| Author(s) : Tsukada H , Nishiyama S , Fukumoto D , Ohba H , Sato K , Kakiuchi T |
| Ref : Synapse , 52 :1 , 2004 |
| Abstract : Tsukada_2004_Synapse_52_1 |
| ESTHER : Tsukada_2004_Synapse_52_1 |
| PubMedSearch : Tsukada_2004_Synapse_52_1 |
| PubMedID: 14755627 |
| Title : Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer's disease - Rinne_2003_J.Neurol.Neurosurg.Psychiatry_74_113 |
| Author(s) : Rinne JO , Kaasinen V , Jarvenpaa T , Nagren K , Roivainen A , Yu M , Oikonen V , Kurki T |
| Ref : Journal of Neurology Neurosurg Psychiatry , 74 :113 , 2003 |
| Abstract : Rinne_2003_J.Neurol.Neurosurg.Psychiatry_74_113 |
| ESTHER : Rinne_2003_J.Neurol.Neurosurg.Psychiatry_74_113 |
| PubMedSearch : Rinne_2003_J.Neurol.Neurosurg.Psychiatry_74_113 |
| PubMedID: 12486280 |
| Title : Synthesis and preliminary evaluation of [18F]FEtP4A, a promising PET tracer for mapping acetylcholinesterase in vivo - Zhang_2002_Nucl.Med.Biol_29_463 |
| Author(s) : Zhang MR , Tsuchiyama A , Haradahira T , Furutsuka K , Yoshida Y , Kida T , Noguchi J , Irie T , Suzuki K |
| Ref : Nucl Med Biol , 29 :463 , 2002 |
| Abstract : Zhang_2002_Nucl.Med.Biol_29_463 |
| ESTHER : Zhang_2002_Nucl.Med.Biol_29_463 |
| PubMedSearch : Zhang_2002_Nucl.Med.Biol_29_463 |
| PubMedID: 12031881 |
| Title : Positron emission tomography: quantitative measurement of brain acetylcholinesterase activity using radiolabeled substrates - Namba_2002_Methods_27_242 |
| Author(s) : Namba H , Fukushi K , Nagatsuka S , Iyo M , Shinotoh H , Tanada S , Irie T |
| Ref : Methods , 27 :242 , 2002 |
| Abstract : Namba_2002_Methods_27_242 |
| ESTHER : Namba_2002_Methods_27_242 |
| PubMedSearch : Namba_2002_Methods_27_242 |
| PubMedID: 12183113 |
| Title : Positron emission tomographic measurement of brain acetylcholinesterase activity using N-[(11)C]methylpiperidin-4-yl acetate without arterial blood sampling: methodology of shape analysis and its diagnostic power for Alzheimer's disease - Tanaka_2001_J.Cereb.Blood.Flow.Metab_21_295 |
| Author(s) : Tanaka N , Fukushi K , Shinotoh H , Nagatsuka S , Namba H , Iyo M , Aotsuka A , Ota T , Tanada S , Irie T |
| Ref : Journal of Cerebral Blood Flow & Metabolism , 21 :295 , 2001 |
| Abstract : Tanaka_2001_J.Cereb.Blood.Flow.Metab_21_295 |
| ESTHER : Tanaka_2001_J.Cereb.Blood.Flow.Metab_21_295 |
| PubMedSearch : Tanaka_2001_J.Cereb.Blood.Flow.Metab_21_295 |
| PubMedID: 11295884 |
| Title : Kinetic analysis of [(11)C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling - Nagatsuka_2001_J.Cereb.Blood.Flow.Metab_21_1354 |
| Author(s) : Nagatsuka Si S , Fukushi K , Shinotoh H , Namba H , Iyo M , Tanaka N , Aotsuka A , Ota T , Tanada S , Irie T |
| Ref : Journal of Cerebral Blood Flow & Metabolism , 21 :1354 , 2001 |
| Abstract : Nagatsuka_2001_J.Cereb.Blood.Flow.Metab_21_1354 |
| ESTHER : Nagatsuka_2001_J.Cereb.Blood.Flow.Metab_21_1354 |
| PubMedSearch : Nagatsuka_2001_J.Cereb.Blood.Flow.Metab_21_1354 |
| PubMedID: 11702050 |
| Title : In-vivo measurements of regional acetylcholine esterase activity in degenerative dementia: comparison with blood flow and glucose metabolism - Herholz_2000_J.Neural.Transm.(Vienna)_107_1457 |
| Author(s) : Herholz K , Bauer B , Wienhard K , Kracht L , Mielke R , Lenz MO , Strotmann T , Heiss WD |
| Ref : J Neural Transm (Vienna) , 107 :1457 , 2000 |
| Abstract : Herholz_2000_J.Neural.Transm.(Vienna)_107_1457 |
| ESTHER : Herholz_2000_J.Neural.Transm.(Vienna)_107_1457 |
| PubMedSearch : Herholz_2000_J.Neural.Transm.(Vienna)_107_1457 |
| PubMedID: 11458998 |
| Title : Human cerebral acetylcholinesterase activity measured with positron emission tomography: procedure, normal values and effect of age - Namba_1999_Eur.J.Nucl.Med_26_135 |
| Author(s) : Namba H , Iyo M , Fukushi K , Shinotoh H , Nagatsuka S , Suhara T , Sudo Y , Suzuki K , Irie T |
| Ref : Eur J Nucl Med , 26 :135 , 1999 |
| Abstract : Namba_1999_Eur.J.Nucl.Med_26_135 |
| ESTHER : Namba_1999_Eur.J.Nucl.Med_26_135 |
| PubMedSearch : Namba_1999_Eur.J.Nucl.Med_26_135 |
| PubMedID: 9933347 |
| Title : Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy - Shinotoh_1999_Ann.Neurol_46_62 |
| Author(s) : Shinotoh H , Namba H , Yamaguchi M , Fukushi K , Nagatsuka S , Iyo M , Asahina M , Hattori T , Tanada S , Irie T |
| Ref : Annals of Neurology , 46 :62 , 1999 |
| Abstract : Shinotoh_1999_Ann.Neurol_46_62 |
| ESTHER : Shinotoh_1999_Ann.Neurol_46_62 |
| PubMedSearch : Shinotoh_1999_Ann.Neurol_46_62 |
| PubMedID: 10401781 |
| Title : N-[11C]methylpiperidine esters as acetylcholinesterase substrates: an in vivo structure-reactivity study - Kilbourn_1998_Nucl.Med.Biol_25_755 |
| Author(s) : Kilbourn MR , Nguyen TB , Snyder SE , Sherman P |
| Ref : Nucl Med Biol , 25 :755 , 1998 |
| Abstract : Kilbourn_1998_Nucl.Med.Biol_25_755 |
| ESTHER : Kilbourn_1998_Nucl.Med.Biol_25_755 |
| PubMedSearch : Kilbourn_1998_Nucl.Med.Biol_25_755 |
| PubMedID: 9863563 |
| Title : Syntheses of carbon-11 labeled piperidine esters as potential in vivo substrates for acetylcholinesterase - Nguyen_1998_Nucl.Med.Biol_25_761 |
| Author(s) : Nguyen TB , Snyder SE , Kilbourn MR |
| Ref : Nucl Med Biol , 25 :761 , 1998 |
| Abstract : Nguyen_1998_Nucl.Med.Biol_25_761 |
| ESTHER : Nguyen_1998_Nucl.Med.Biol_25_761 |
| PubMedSearch : Nguyen_1998_Nucl.Med.Biol_25_761 |
| PubMedID: 9863564 |
| Title : Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease - Iyo_1997_Lancet_349_1805 |
| Author(s) : Iyo M , Namba H , Fukushi K , Shinotoh H , Nagatsuka S , Suhara T , Sudo Y , Suzuki K , Irie T |
| Ref : Lancet , 349 :1805 , 1997 |
| Abstract : Iyo_1997_Lancet_349_1805 |
| ESTHER : Iyo_1997_Lancet_349_1805 |
| PubMedSearch : Iyo_1997_Lancet_349_1805 |
| PubMedID: 9269216 |
| Title : Brain acetylcholinesterase activity: validation of a PET tracer in a rat model of Alzheimer's disease - Irie_1996_J.Nucl.Med_37_649 |
| Author(s) : Irie T , Fukushi K , Namba H , Iyo M , Tamagami H , Nagatsuka S , Ikota N |
| Ref : J Nucl Med , 37 :649 , 1996 |
| Abstract : Irie_1996_J.Nucl.Med_37_649 |
| ESTHER : Irie_1996_J.Nucl.Med_37_649 |
| PubMedSearch : Irie_1996_J.Nucl.Med_37_649 |
| PubMedID: 8691261 |