N-Methylnaloxonium
General
Type : Drug || Isoquinoline
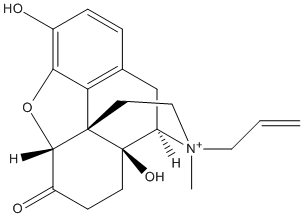
Chemical_Nomenclature : (3S,4R,4aS,7aR,12bS)-4a,9-dihydroxy-3-methyl-3-prop-2-enyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-3-ium-7-one
Canonical SMILES : C[N+]1(CCC23C4C(=O)CCC2(C1CC5=C3C(=C(C=C5)O)O4)O)CC=C
InChI : InChI=1S\/C20H23NO4\/c1-3-9-21(2)10-8-19-16-12-4-5-13(22)17(16)25-18(19)14(23)6-7-20(19,24)15(21)11-12\/h3-5,15,18,24H,1,6-11H2,2H3\/p+1\/t15-,18+,19+,20-,21-\/m1\/s1
InChIKey : PCSQOABIHJXZMR-YNUHATHGSA-O || ICONPJDAXITIPI-UXYWFNEESA-N
Other name(s) : (5A,17R)-4,5-epoxy-3,14-dihydroxy-17-methyl-6-oxo-17-(2-propenyl)-morphinanium, DB04509, Naloxone methiodide, N-Methylnaloxonium iodide, 93302-47-7, MLS001334009, (5alpha,17R)-4,5-Epoxy-3,14-dihydroxy-17-methyl-6-oxo-17-(2-propenyl)-morphinanium iodide, SMR000875254
MW : 342.40
Formula : C20H24NO4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carb_B_Chordata
References (1)
| Title : Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme - Bencharit_2003_Nat.Struct.Biol_10_349 |
| Author(s) : Bencharit S , Morton CL , Xue Y , Potter PM , Redinbo MR |
| Ref : Nat Struct Biol , 10 :349 , 2003 |
| Abstract : Bencharit_2003_Nat.Struct.Biol_10_349 |
| ESTHER : Bencharit_2003_Nat.Struct.Biol_10_349 |
| PubMedSearch : Bencharit_2003_Nat.Struct.Biol_10_349 |
| PubMedID: 12679808 |
| Gene_locus related to this paper: human-CES1 |