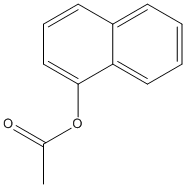
1-Naphtylacetate
General
Type : Acetate || Naphtyl ester || Aromatic ester (two rings) || Naphthalen
Chemical_Nomenclature : naphthalen-1-yl acetate
Canonical SMILES : CC(=O)OC1=CC=CC2=CC=CC=C21
InChI : InChI=1S\/C12H10O2\/c1-9(13)14-12-8-4-6-10-5-2-3-7-11(10)12\/h2-8H,1H3
InChIKey : VGKONPUVOVVNSU-UHFFFAOYSA-N
Other name(s) : 1-Naphthyl acetate, 1-Acetoxynaphthalene, Alpha-Naphthyl acetate, 1-Naphthol, acetate, A-Naphthyl acetate, Acetic acid 1-naphthyl ester, C2-naphthyl ester, SCHEMBL76633, CHEMBL2286101, alpha-NA
References (13)
| Title : Identification and characterization of an acetyl xylan esterase from Aspergillus oryzae - Kato_2021_J.Biosci.Bioeng__ |
| Author(s) : Kato T , Shiono Y , Koseki T |
| Ref : J Biosci Bioeng , : , 2021 |
| Abstract : Kato_2021_J.Biosci.Bioeng__ |
| ESTHER : Kato_2021_J.Biosci.Bioeng__ |
| PubMedSearch : Kato_2021_J.Biosci.Bioeng__ |
| PubMedID: 34376338 |
| Gene_locus related to this paper: aspfu-faec , aspor-faec |
| Title : 1-Naphthyl acetate: a chromogenic substrate for the detection of erythrocyte acetylcholinesterase activity - Chowdhary_2018_Biochimie_154_194 |
| Author(s) : Chowdhary S , Bhattacharyya R , Banerjee D |
| Ref : Biochimie , 154 :194 , 2018 |
| Abstract : Chowdhary_2018_Biochimie_154_194 |
| ESTHER : Chowdhary_2018_Biochimie_154_194 |
| PubMedSearch : Chowdhary_2018_Biochimie_154_194 |
| PubMedID: 30201403 |
| Title : An extended loop in CE7 carbohydrate esterase family is dispensable for oligomerization but required for activity and thermostability - Singh_2016_J.Struct.Biol_194_434 |
| Author(s) : Singh MK , Manoj N |
| Ref : J Struct Biol , 194 :434 , 2016 |
| Abstract : Singh_2016_J.Struct.Biol_194_434 |
| ESTHER : Singh_2016_J.Struct.Biol_194_434 |
| PubMedSearch : Singh_2016_J.Struct.Biol_194_434 |
| PubMedID: 27085421 |
| Gene_locus related to this paper: thema-TM0077 |
| Title : Characterization of a novel lipolytic enzyme from Aspergillus oryzae - Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| Author(s) : Koseki T , Asai S , Saito N , Mori M , Sakaguchi Y , Ikeda K , Shiono Y |
| Ref : Applied Microbiology & Biotechnology , 97 :5351 , 2013 |
| Abstract : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| ESTHER : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| PubMedSearch : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| PubMedID: 23001008 |
| Gene_locus related to this paper: aspor-q2uf27 |
| Title : An inserted alpha\/beta subdomain shapes the catalytic pocket of Lactobacillus johnsonii cinnamoyl esterase - Lai_2011_PLoS.One_6_e23269 |
| Author(s) : Lai KK , Stogios PJ , Vu C , Xu X , Cui H , Molloy S , Savchenko A , Yakunin A , Gonzalez CF |
| Ref : PLoS ONE , 6 :e23269 , 2011 |
| Abstract : Lai_2011_PLoS.One_6_e23269 |
| ESTHER : Lai_2011_PLoS.One_6_e23269 |
| PubMedSearch : Lai_2011_PLoS.One_6_e23269 |
| PubMedID: 21876742 |
| Gene_locus related to this paper: lacjo-q74hk5 |
| Title : Carboxylesterase activity, cDNA sequence, and gene expression in malathion susceptible and resistant strains of the cotton aphid, Aphis gossypii - Pan_2009_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_152_266 |
| Author(s) : Pan Y , Guo H , Gao X |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 152 :266 , 2009 |
| Abstract : Pan_2009_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_152_266 |
| ESTHER : Pan_2009_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_152_266 |
| PubMedSearch : Pan_2009_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_152_266 |
| PubMedID: 19110065 |
| Gene_locus related to this paper: aphgo-cxest |
| Title : Isolation, characterization, and heterologous expression of a carboxylesterase of Pseudomonas aeruginosa PAO1 - Pesaresi_2005_Curr.Microbiol_50_102 |
| Author(s) : Pesaresi A , Devescovi G , Lamba D , Venturi V , Degrassi G |
| Ref : Curr Microbiol , 50 :102 , 2005 |
| Abstract : Pesaresi_2005_Curr.Microbiol_50_102 |
| ESTHER : Pesaresi_2005_Curr.Microbiol_50_102 |
| PubMedSearch : Pesaresi_2005_Curr.Microbiol_50_102 |
| PubMedID: 15717224 |
| Gene_locus related to this paper: pseae-PA3859 |
| Title : Cloning and characterization of EstC from Burkholderia gladioli, a novel-type esterase related to plant enzymes - Reiter_2000_Appl.Microbiol.Biotechnol_54_778 |
| Author(s) : Reiter B , Glieder A , Talker D , Schwab H |
| Ref : Applied Microbiology & Biotechnology , 54 :778 , 2000 |
| Abstract : Reiter_2000_Appl.Microbiol.Biotechnol_54_778 |
| ESTHER : Reiter_2000_Appl.Microbiol.Biotechnol_54_778 |
| PubMedSearch : Reiter_2000_Appl.Microbiol.Biotechnol_54_778 |
| PubMedID: 11152069 |
| Gene_locus related to this paper: burgl-EstC |
| Title : An improved method for butyrylcholinesterase phenotyping [published erratum appears in Biochem Genet 1994 Oct\;32(9-10):379] - Picheth_1994_Biochem.Genet_32_83 |
| Author(s) : Picheth G , Fadel-Picheth C , Primo-Parmo SL , Chautard-Freire-Maia EA , Vieira MM |
| Ref : Biochemical Genetics , 32 :83 , 1994 |
| Abstract : Picheth_1994_Biochem.Genet_32_83 |
| ESTHER : Picheth_1994_Biochem.Genet_32_83 |
| PubMedSearch : Picheth_1994_Biochem.Genet_32_83 |
| PubMedID: 7980387 |
| Title : [Multiple molecular forms of esterases from grass aphids inhibitory identification and stereospecificity] - Volkova_1983_Biokhimiia_48_1634 |
| Author(s) : Volkova RI , Titova EV |
| Ref : Biokhimiia , 48 :1634 , 1983 |
| Abstract : Volkova_1983_Biokhimiia_48_1634 |
| ESTHER : Volkova_1983_Biokhimiia_48_1634 |
| PubMedSearch : Volkova_1983_Biokhimiia_48_1634 |
| PubMedID: 6639987 |
| Title : Prediction of drug sensitivity in individuals with atypical serum cholinesterase based on in vitro biochemical studies - Valentino_1981_Biochem.Pharmacol_30_1643 |
| Author(s) : Valentino RJ , Lockridge O , Eckerson HW , La Du BN |
| Ref : Biochemical Pharmacology , 30 :1643 , 1981 |
| Abstract : Valentino_1981_Biochem.Pharmacol_30_1643 |
| ESTHER : Valentino_1981_Biochem.Pharmacol_30_1643 |
| PubMedSearch : Valentino_1981_Biochem.Pharmacol_30_1643 |
| PubMedID: 7271851 |
| Title : The use of naphthyl esters as substrates in esterase determinations - |
| Author(s) : Augustinsson KB |
| Ref : Biochimica & Biophysica Acta , 159 :197 , 1968 |
| PubMedID: 5650435 |