Trimedoxime
General
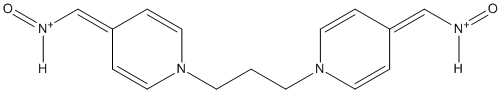
Type : Oxime, Bisoxime, Bispyridinium
Chemical_Nomenclature : oxo-[[1-[3-[4-(oxoazaniumylmethylidene)pyridin-1-yl]propyl]pyridin-4-ylidene]methyl]azanium\;dibromide
Canonical SMILES : C1=CN(C=CC1=C[NH+]=O)CCCN2C=CC(=C[NH+]=O)C=C2.[Br-].[Br-]
InChI : InChI=1S\/C15H16N4O2.2BrH\/c20-16-12-14-2-8-18(9-3-14)6-1-7-19-10-4-15(5-11-19)13-17-21\;\;\/h2-5,8-13H,1,6-7H2\;2*1H
InChIKey : JHZHWVQTOXIXIV-UHFFFAOYSA-N, LJYGXPCCGGSATE-UHFFFAOYSA-P
Other name(s) : TMB-4, TMB4, Trimedoxime, Dipyroxime, Dipiroxim, Trimedoxime N, Dipiroxim dibromide, BRN 1552160, 6736-02-3, Pyridinium, 1,1'-(1,3-propanediyl)bis(4-(hydroxyimino)methyl-, oxo-[[1-[3-[4-(oxoazaniumylmethylidene)pyridin-1-yl]propyl]pyridin-4-ylidene]methyl]azanium\;dibromide
MW : 446.137
Formula : C15H18Br2N4O2
CAS_number : 56-97-3
CID PubChem :
InChIKey UniChem :
Iuphar :
Wikipedia : Trimedoxime_bromide
Target
Structure : No structure
Families : No family
References
No reference