Tacrine-pyridinealdoxime-1
General
Type : Oxime, Pyridine-aldoxime, Uncharged Oxime, Derivative of Tacrine, Acridine
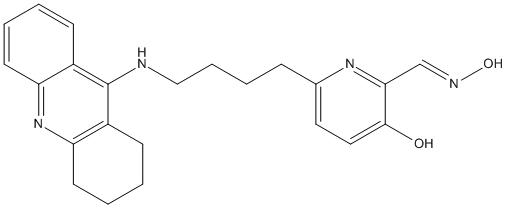
Chemical_Nomenclature : 7.2.8. 2-[1-(Hydroxyimino)methyl]-6-{4-[(1,2,3,4-tetrahydroacridin-9-yl)amino]butyl}pyridin-3-ol, (2E)-2-[(hydroxyamino)methylidene]-6-[4-(1,2,3,4-tetrahydroacridin-9-ylamino)butyl]pyridin-3-one
Canonical SMILES : C3CCC2=NC1=CC=CC=C1C(=C2C3)NCCCCC4=NC(=CNO)C(=O)C=C4 || ON=Cc1nc(CCCCNc2c3CCCCc3nc4ccccc24)ccc1O || C1CCC2=NC3=CC=CC=C3C(=C2C1)NCCCCC4=NC(=CNO)C(=O)C=C4
InChI : InChI=1S\/C23H26N4O2\/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23\/h1,3,8,10,12-13,15,25,29H,2,4-7,9,11,14H2,(H,24,27), InChI=1S\/C23H26N4O2\/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23\/h1,3,8,10,12-13,15,28-29H,2,4-7,9,11,14H2,(H,24,27)\/b25-15+, InChI=1S\/C23H26N4O2\/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23\/h1,3,8,10,12-13,15,25,29H,2,4-7,9,11,14H2,(H,24,27)\/b21-15+
InChIKey : YQHRRIODIOINAV-UHFFFAOYSA-N, WBLSVMZKFSZDDW-MFKUBSTISA-N, YQHRRIODIOINAV-RCCKNPSSSA-N
Other name(s) : CHEMBL3234588, ZINC000169310262, SCHEMBL16712057, SCHEMBL18487408
MW :
Formula : C23H27N4O2
CAS_number :
CID PubChem :
InChIKey UniChem :
Iuphar :
Wikipedia :
References
No reference