RS170B
General
Type : Oxime, Carboxamide
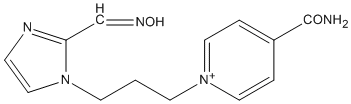
Chemical_Nomenclature : 1-[3-[(2E)-2-(nitrosomethylidene)-1H-imidazol-3-yl]propyl]pyridin-1-ium-4-carboxamide
Canonical SMILES : C1=C[N+](=CC=C1C(=O)N)CCCN2C=CNC2=CN=O
InChI : InChI=1S\/C13H15N5O2\/c14-13(19)11-2-7-17(8-3-11)5-1-6-18-9-4-15-12(18)10-16-20\/h2-4,7-10H,1,5-6H2,(H2-,14,15,19,20)\/p+1
InChIKey : AEYBMKVDFNSVJO-UHFFFAOYSA-O
Other name(s) : RS-170B, SCHEMBL16664108, 4-Carbamoyl-1-(3-{2-[(hydroxyimino)methyl]imidazol-1-yl}propyl)pyridin-1-ium Bromide, CHEMBL3139900
MW : 274.3
Formula : C13H16N5O2
CAS_number :
CID PubChem :
InChIKey UniChem :
Iuphar :
Wikipedia :
References
No reference