QMP-C8
General
Type : non-oxime, Quinone methide precursor
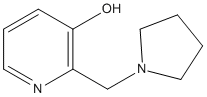
Chemical_Nomenclature : 2-(pyrrolidin-1-ylmethyl)pyridin-3-ol
Canonical SMILES : C1CCN(C1)CC2=C(C=CC=N2)O
InChI : InChI=1S\/C10H14N2O\/c13-10-4-3-5-11-9(10)8-12-6-1-2-7-12\/h3-5,13H,1-2,6-8H2
InChIKey : JUNVYXJFFGIRNO-UHFFFAOYSA-N
Other name(s) : 2-(pyrrolidin-1-ylmethyl)pyridin-3-ol, NSC72099, AC1L5K3N, AC1Q4WY9, AC1Q78CM
MW : 178.23
Formula : C10H14N2O
CAS_number : 7149-45-3
CID PubChem :
InChIKey UniChem :
Iuphar :
Wikipedia :
Target
Structure : No structure
Families : No family
References
No reference