K127
General
Type : Oxime, Bispyridinium
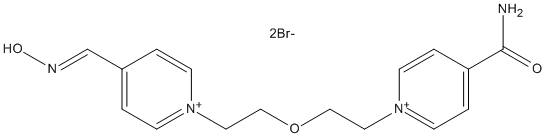
Chemical_Nomenclature :
Canonical SMILES : C1(=CC=[N+](C=C1)CCOCC[N+]2=CC=C(C=C2)C(=O)N([H])[H])C(=NO[H])[H]
InChI : InChI=1S\/C16H18N4O3\/c17-16(21)15-3-7-20(8-4-15)10-12-23-11-9-19-5-1-14(2-6-19)13-18-22\/h1-8,13H,9-12H2,(H-,17,21)\/p+2
InChIKey : OVVOCKYUHCQSBH-UHFFFAOYSA-P
Other name(s) : 1-(4-carbamoylpyridinium)-5-(4-hydroxyimino-methylpyridinium)-3-oxapentane dibromide
MW : 476,16
Formula : C16H20Br2N4O3
CAS_number :
CID PubChem :
InChIKey UniChem :
Iuphar :
Wikipedia :
Target
Structure : No structure
Families : No family
References
No reference