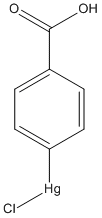
p-chloromercuribenzoic-acid
Alkylating reagent commonly used to irreversibly label cysteines in biomolecules. Covalent modification by iodoacetamide at Cys-209 of Ag85C inactivates the enzyme
General
Type : Thiol-reactive
Chemical_Nomenclature : (4-carboxyphenyl)-chloromercury
Canonical SMILES : C1=CC(=CC=C1C(=O)O)[Hg]Cl
InChI : InChI=1S\/C7H5O2.ClH.Hg\/c8-7(9)6-4-2-1-3-5-6\;\;\/h2-5H,(H,8,9)\;1H\;\/q\;\;+1\/p-1
InChIKey : YFZOUMNUDGGHIW-UHFFFAOYSA-M
Other name(s) : 4-chloromercuribenzoic acid,P-Chloromercuribenzoic acid,PCMB,P-Chloromercurybenzoic acid,P-(chloromercuri)benzoic acid,P-Chloromercuric benzoic acid
MW : 357.15
Formula : C7H5ClHgO2
CAS_number :
PubChem : 1730
UniChem : YFZOUMNUDGGHIW-UHFFFAOYSA-M
IUPHAR :
Wikipedia :
Target
Families : p-chloromercuribenzoic-acid ligand of proteins in family: A85-Mycolyl-transferase
Stucture : 4QDO Crystal structure of Ag85C co-crystallized with p-chloromercuribenzoic acid
Protein : myctu-a85c
References (4)
| Title : Inactivation of the Mycobacterium tuberculosis antigen 85 complex by covalent, allosteric inhibitors - Favrot_2014_J.Biol.Chem_289_25031 |
| Author(s) : Favrot L , Lajiness DH , Ronning DR |
| Ref : Journal of Biological Chemistry , 289 :25031 , 2014 |
| Abstract : Favrot_2014_J.Biol.Chem_289_25031 |
| ESTHER : Favrot_2014_J.Biol.Chem_289_25031 |
| PubMedSearch : Favrot_2014_J.Biol.Chem_289_25031 |
| PubMedID: 25028518 |
| Gene_locus related to this paper: myctu-a85c |
| Title : The effects of p-chloromercuribenzoate on muscarinic receptors in the cerebral cortex - Birdsall_1983_Br.J.Pharmacol_80_187 |
| Author(s) : Birdsall NJ , Burgen AS , Hulme EC , Wong EH |
| Ref : British Journal of Pharmacology , 80 :187 , 1983 |
| Abstract : Birdsall_1983_Br.J.Pharmacol_80_187 |
| ESTHER : Birdsall_1983_Br.J.Pharmacol_80_187 |
| PubMedSearch : Birdsall_1983_Br.J.Pharmacol_80_187 |
| PubMedID: 6652369 |
| Title : The effect of p-chloromercuribenzoate on structure-binding relationships of muscarinic receptors in the rat cerebral cortex - Birdsall_1983_Br.J.Pharmacol_80_197 |
| Author(s) : Birdsall NJ , Burgen AS , Hulme EC , Wong EH |
| Ref : British Journal of Pharmacology , 80 :197 , 1983 |
| Abstract : Birdsall_1983_Br.J.Pharmacol_80_197 |
| ESTHER : Birdsall_1983_Br.J.Pharmacol_80_197 |
| PubMedSearch : Birdsall_1983_Br.J.Pharmacol_80_197 |
| PubMedID: 6652370 |
| Title : Effects of p-chloromercuribenzoate on muscarinic receptor binding in rat brain [proceedings] - |
| Author(s) : Birdsall NJ , Burgen AS , Hulme EC , Wong EH |
| Ref : British Journal of Pharmacology , 68 :142P , 1980 |
| PubMedID: 7357157 |