diEt-PFP
General
Type : Organophosphate
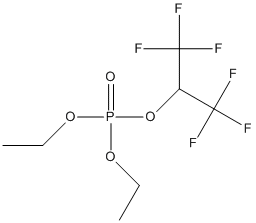
Chemical_Nomenclature : diethyl 1,1,1,3,3,3-hexafluoropropan-2-yl phosphate
Canonical SMILES : CCOP(=O)(OCC)OC(C(F)(F)F)C(F)(F)F
InChI : InChI=1S\/C7H11F6O4P\/c1-3-15-18(14,16-4-2)17-5(6(8,9)10)7(11,12)13\/h5H,3-4H2,1-2H3
InChIKey : YEMNWQQLKIIPRQ-UHFFFAOYSA-N
Other name(s) : ZINC05718302,Phosphoric acid diethyl=1-(trifluoromethyl)-2,2,2-trifluoroethyl ester,2-diethoxyphosphoryloxy-1,1,1,3,3,3-hexafluoro-propane
MW : 304.12
Formula : C7H11F6O4P
CAS_number :
PubChem : 12513073
UniChem : YEMNWQQLKIIPRQ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics - Makhaeva_2009_Bioorg.Med.Chem.Lett_19_5528 |
| Author(s) : Makhaeva GF , Aksinenko AY , Sokolov VB , Serebryakova OG , Richardson RJ |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :5528 , 2009 |
| Abstract : Makhaeva_2009_Bioorg.Med.Chem.Lett_19_5528 |
| ESTHER : Makhaeva_2009_Bioorg.Med.Chem.Lett_19_5528 |
| PubMedSearch : Makhaeva_2009_Bioorg.Med.Chem.Lett_19_5528 |
| PubMedID: 19717305 |
| Title : Esterase profile and analysis of structure-inhibitor selectivity relationships for homologous phosphorylated 1-hydroperfluoroisopropanols - |
| Author(s) : Makhaeva GF , Serebryakova OG , Boltneva NP , Galenko TG , Aksinenko AY , Sokolov VB , Martynov IV |
| Ref : Dokl Biochem Biophys , 423 :352 , 2008 |
| PubMedID: 19230387 |