Tz2Pa5-syn1
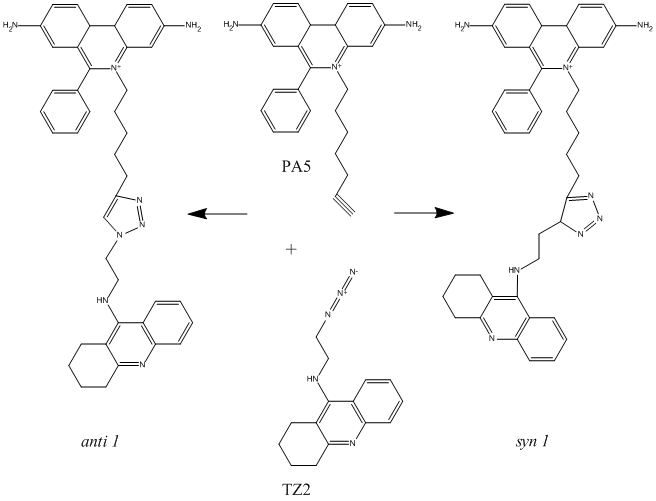
The AChE inhibitor TZ2PA5 results from 1,3-dipolar cycloaddition (click chemistry) between a PAS inhibitor (phenylphenanthridinium) equipped with an acetylene group at the end of a flexible methylene chain and an active site inhibitor (tacrine)equipped with an azide group at the end of a methylene chain
General
Type : Acridine,Phenanthridinium,Derivative of Tacrine,Alkyl linked bis-ligand,Triazol
Chemical_Nomenclature : 6-phenyl-5-[5-[3-[2-(1,2,3,4-tetrahydroacridin-9-ylamino)ethyl]triazol-4-yl]pentyl]phenanthridin-5-ium-3,8-diamine
Canonical SMILES : C1CCC2=NC3=CC=CC=C3C(=C2C1)NCCN4C(=CN=N4)CCCCC[N+]5=C6C=C(C=CC6=C7C=CC(=CC7=C5C8=CC=CC=C8)N)N
InChI : InChI=1S\/C41H42N8\/c42-29-18-20-32-33-21-19-30(43)26-39(33)48(41(36(32)25-29)28-11-3-1-4-12-28)23-10-2-5-13-31-27-45-47-49(31)24-22-44-40-34-14-6-8-16-37(34)46-38-17-9-7-15-35(38)40\/h1,3-4,6,8,11-12,14,16,18-21,25-27,43H,2,5,7,9-10,13,15,17,22-24,42H2,(H,44,46)\/p+1
InChIKey : ALUZXISFDWHPFA-UHFFFAOYSA-O
Other name(s) : 3,8-Diamino-6-Phenyl-5-[6-[1-[2-[(1,2,3,4-tetrahydro-9-acridinyl)amino]ethyl]-1H-1,2,3-triazol-5-yl]pentyl] phenanthridinium,5NZ,6-Phenyl-5-[5-[3-[2-(1,2,3,4-Tetrahydroacridin-9-Ylamino)ethyl]-1,2,3-Triazol-4-Yl]pentyl]phenanthridin-5-Ium-3,8-Diamine
MW :
Formula : C41H43N8
CAS_number :
PubChem : 11193070
UniChem : ALUZXISFDWHPFA-UHFFFAOYSA-O
IUPHAR :
Wikipedia :
Target
References (5)
| Title : Steric and Dynamic Parameters Influencing In Situ Cycloadditions to Form Triazole Inhibitors with Crystalline Acetylcholinesterase - Bourne_2016_J.Am.Chem.Soc_138_1611 |
| Author(s) : Bourne Y , Sharpless KB , Taylor P , Marchot P |
| Ref : Journal of the American Chemical Society , 138 :1611 , 2016 |
| Abstract : Bourne_2016_J.Am.Chem.Soc_138_1611 |
| ESTHER : Bourne_2016_J.Am.Chem.Soc_138_1611 |
| PubMedSearch : Bourne_2016_J.Am.Chem.Soc_138_1611 |
| PubMedID: 26731630 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : In situ click chemistry: enzyme inhibitors made to their own specifications - Manetsch_2004_J.Am.Chem.Soc_126_12809 |
| Author(s) : Manetsch R , Krasinski A , Radic Z , Raushel J , Taylor P , Sharpless KB , Kolb HC |
| Ref : Journal of the American Chemical Society , 126 :12809 , 2004 |
| Abstract : Manetsch_2004_J.Am.Chem.Soc_126_12809 |
| ESTHER : Manetsch_2004_J.Am.Chem.Soc_126_12809 |
| PubMedSearch : Manetsch_2004_J.Am.Chem.Soc_126_12809 |
| PubMedID: 15469276 |
| Title : Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation - Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| Author(s) : Bourne Y , Kolb HC , Radic Z , Sharpless KB , Taylor P , Marchot P |
| Ref : Proc Natl Acad Sci U S A , 101 :1449 , 2004 |
| Abstract : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| ESTHER : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| PubMedSearch : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| PubMedID: 14757816 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Cover picture: angew. Chem. Int. Ed. 6\/2002 - Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| Author(s) : Lewis WG , Green LG , Grynszpan F , Radic Z , Carlier PR , Taylor P , Finn MG , Sharpless KB |
| Ref : Angew Chem Int Ed Engl , 41 :875 , 2002 |
| Abstract : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| ESTHER : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| PubMedSearch : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| PubMedID: |
| Title : Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks - |
| Author(s) : Lewis WG , Green LG , Grynszpan F , Radic Z , Carlier PR , Taylor P , Finn MG , Sharpless KB |
| Ref : Angew Chem Int Ed Engl , 41 :1053 , 2002 |
| PubMedID: 12491310 |