Tyramine
An indirect sympathomimetic. Tyramine does not directly activate adrenergic receptors, but it can serve as a substrate for adrenergic uptake systems and monoamine oxidase so it prolongs the actions of adrenergic transmitters. It also provokes transmitter release from adrenergic terminals. Tyramine may be a neurotransmitter in some invertebrate nervous systems.
General
Type : Adrenergic Uptake Inhibitors,Not A\/B H target,Drug
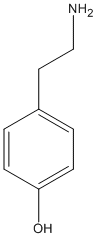
Chemical_Nomenclature : 4-(2-aminoethyl)phenol
Canonical SMILES : C1=CC(=CC=C1CCN)O
InChI : InChI=1S\/C8H11NO\/c9-6-5-7-1-3-8(10)4-2-7\/h1-4,10H,5-6,9H2
InChIKey : DZGWFCGJZKJUFP-UHFFFAOYSA-N
Other name(s) : Tyramin,Tyrosamine,Systogene,Uteramine,Tocosine,p-Tyramine,Tenosin-wirkstoff,Tyramine base,p-Hydroxyphenethylamine
MW : 137.179
Formula : C8H11NO
CAS_number :
PubChem : 5610
UniChem : DZGWFCGJZKJUFP-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Tyramine
Target
References (4)
| Title : Serum butyrylcholinesterase of non-human primate shows amine sensitive aryl acyl amidase and metallopeptidase activities and characteristics similar to those of the human serum enzyme - Bhanumathy_1998_Indian.J.Biochem.Biophys_35_148 |
| Author(s) : Bhanumathy CD , Rao RV , Balasubramanian AS |
| Ref : Indian J Biochem Biophys , 35 :148 , 1998 |
| Abstract : Bhanumathy_1998_Indian.J.Biochem.Biophys_35_148 |
| ESTHER : Bhanumathy_1998_Indian.J.Biochem.Biophys_35_148 |
| PubMedSearch : Bhanumathy_1998_Indian.J.Biochem.Biophys_35_148 |
| PubMedID: 9803663 |
| Title : Cholinesterases exhibiting aryl acylamidase activity in human amniotic fluid - Jayanthi_1992_Clin.Chim.Acta_205_157 |
| Author(s) : Jayanthi LD , Balasubramanian N , Balasubramanian AS |
| Ref : Clinica Chimica Acta , 205 :157 , 1992 |
| Abstract : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| ESTHER : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| PubMedSearch : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| PubMedID: 1349516 |
| Title : Human cerebrospinal fluid acetylcholinesterase and butyrylcholinesterase. Evidence for identity between the serum and cerebrospinal fluid butyrylcholinesterase - Rao_1989_Clin.Chim.Acta_183_135 |
| Author(s) : Rao RV , Gnanamuthu C , Balasubramanian AS |
| Ref : Clinica Chimica Acta , 183 :135 , 1989 |
| Abstract : Rao_1989_Clin.Chim.Acta_183_135 |
| ESTHER : Rao_1989_Clin.Chim.Acta_183_135 |
| PubMedSearch : Rao_1989_Clin.Chim.Acta_183_135 |
| PubMedID: 2791303 |
| Title : The aryl acylamidases and their relationship to cholinesterases in human serum, erythrocyte and liver - George_1981_Eur.J.Biochem_121_177 |
| Author(s) : George ST , Balasubramanian AS |
| Ref : European Journal of Biochemistry , 121 :177 , 1981 |
| Abstract : George_1981_Eur.J.Biochem_121_177 |
| ESTHER : George_1981_Eur.J.Biochem_121_177 |
| PubMedSearch : George_1981_Eur.J.Biochem_121_177 |
| PubMedID: 7035166 |