Tri-o-cresylphosphate
metabolic precursor to a neuropathy target esterase (NTE, PNPLA6) inhibitor. TRI-O-CBESYL PHOSPHATE (TOCP) is metabolized in vitro and in vivo to form potent esterase inhibitors. CBDP and Phenyl-BDPOPhenyl-BDPO are potent NTE inhibitors
General
Type : Organophosphate,Organophosphorus flame retardant plasticizer,OPIDN-OP
Chemical_Nomenclature : tris(2-methylphenyl) phosphate
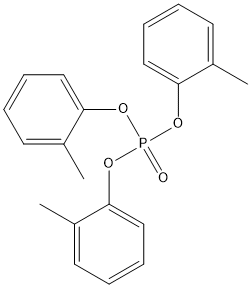
Canonical SMILES : CC1=CC=CC=C1OP(=O)(OC2=CC=CC=C2C)OC3=CC=CC=C3C
InChI : InChI=1S\/C21H21O4P\/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3\/h4-15H,1-3H3
InChIKey : YSMRWXYRXBRSND-UHFFFAOYSA-N
Other name(s) : Tri-o-cresyl phosphate,Triorthocresyl phosphate,Phosphoric acid tris(2-methylphenyl) ester,Tri-o-tolyl phosphate,tri-o-tolylphosphate,o-Tolyl phosphate,TOCP,TOTP,tri-cresyl phosphate,o-Cresyl phosphate,Phosflex 179C,Phosphoric acid, tri-o-tolyl ester,Phosphoric acid, tris(2-methylphenyl) ester,TCP,TOFK,tri-ortho-cresyl phosphate
MW : 368.37
Formula : C21H21O4P
CAS_number : 78-30-8
PubChem : 6527
UniChem : YSMRWXYRXBRSND-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (47)
| Title : Tricresyl phosphate isomers exert estrogenic effects via G protein-coupled estrogen receptor-mediated pathways - Ji_2020_Environ.Pollut_264_114747 |
| Author(s) : Ji X , Li N , Ma M , Rao K , Yang R , Wang Z |
| Ref : Environ Pollut , 264 :114747 , 2020 |
| Abstract : Ji_2020_Environ.Pollut_264_114747 |
| ESTHER : Ji_2020_Environ.Pollut_264_114747 |
| PubMedSearch : Ji_2020_Environ.Pollut_264_114747 |
| PubMedID: 32559878 |
| Title : Assessment of neurotoxic effects of tri-cresyl phosphates (TCPs) and cresyl saligenin phosphate (CBDP) using a combination of in vitro techniques - Hausherr_2017_Neurotoxicol_59_210 |
| Author(s) : Hausherr V , Schobel N , Liebing J , van Thriel C |
| Ref : Neurotoxicology , 59 :210 , 2017 |
| Abstract : Hausherr_2017_Neurotoxicol_59_210 |
| ESTHER : Hausherr_2017_Neurotoxicol_59_210 |
| PubMedSearch : Hausherr_2017_Neurotoxicol_59_210 |
| PubMedID: 27288108 |
| Title : Cresyl saligenin phosphate makes multiple adducts on free histidine, but does not form an adduct on histidine 438 of human butyrylcholinesterase - Liyasova_2013_Chem.Biol.Interact_203_103 |
| Author(s) : Liyasova MS , Schopfer LM , Lockridge O |
| Ref : Chemico-Biological Interactions , 203 :103 , 2013 |
| Abstract : Liyasova_2013_Chem.Biol.Interact_203_103 |
| ESTHER : Liyasova_2013_Chem.Biol.Interact_203_103 |
| PubMedSearch : Liyasova_2013_Chem.Biol.Interact_203_103 |
| PubMedID: 22898212 |
| Title : Effects of viscosity and osmotic stress on the reaction of human butyrylcholinesterase with cresyl saligenin phosphate, a toxicant related to aerotoxic syndrome: kinetic and molecular dynamics studies - Masson_2013_Biochem.J_454_387 |
| Author(s) : Masson P , Lushchekina SV , Schopfer LM , Lockridge O |
| Ref : Biochemical Journal , 454 :387 , 2013 |
| Abstract : Masson_2013_Biochem.J_454_387 |
| ESTHER : Masson_2013_Biochem.J_454_387 |
| PubMedSearch : Masson_2013_Biochem.J_454_387 |
| PubMedID: 23782236 |
| Title : Cresyl saligenin phosphate, an organophosphorus toxicant, makes covalent adducts with histidine, lysine, and tyrosine residues of human serum albumin - Liyasova_2012_Chem.Res.Toxicol_25_1752 |
| Author(s) : Liyasova MS , Schopfer LM , Lockridge O |
| Ref : Chemical Research in Toxicology , 25 :1752 , 2012 |
| Abstract : Liyasova_2012_Chem.Res.Toxicol_25_1752 |
| ESTHER : Liyasova_2012_Chem.Res.Toxicol_25_1752 |
| PubMedSearch : Liyasova_2012_Chem.Res.Toxicol_25_1752 |
| PubMedID: 22793878 |
| Title : Differential sensitivity of plasma carboxylesterase-null mice to parathion, chlorpyrifos and chlorpyrifos oxon, but not to diazinon, dichlorvos, diisopropylfluorophosphate, cresyl saligenin phosphate, cyclosarin thiocholine, tabun thiocholine, and carbofuran - Duysen_2012_Chem.Biol.Interact_195_189 |
| Author(s) : Duysen EG , Cashman JR , Schopfer LM , Nachon F , Masson P , Lockridge O |
| Ref : Chemico-Biological Interactions , 195 :189 , 2012 |
| Abstract : Duysen_2012_Chem.Biol.Interact_195_189 |
| ESTHER : Duysen_2012_Chem.Biol.Interact_195_189 |
| PubMedSearch : Duysen_2012_Chem.Biol.Interact_195_189 |
| PubMedID: 22209767 |
| Title : Reaction of cresyl saligenin phosphate, the organophosphorus agent implicated in aerotoxic syndrome, with human cholinesterases: mechanistic studies employing kinetics, mass spectrometry, and X-ray structure analysis - Carletti_2011_Chem.Res.Toxicol_24_797 |
| Author(s) : Carletti E , Schopfer LM , Colletier JP , Froment MT , Nachon F , Weik M , Lockridge O , Masson P |
| Ref : Chemical Research in Toxicology , 24 :797 , 2011 |
| Abstract : Carletti_2011_Chem.Res.Toxicol_24_797 |
| ESTHER : Carletti_2011_Chem.Res.Toxicol_24_797 |
| PubMedSearch : Carletti_2011_Chem.Res.Toxicol_24_797 |
| PubMedID: 21438623 |
| Gene_locus related to this paper: human-BCHE |
| Title : Induction of plasma acetylcholinesterase activity in mice challenged with organophosphorus poisons - Duysen_2011_Toxicol.Appl.Pharmacol_255_214 |
| Author(s) : Duysen EG , Lockridge O |
| Ref : Toxicol Appl Pharmacol , 255 :214 , 2011 |
| Abstract : Duysen_2011_Toxicol.Appl.Pharmacol_255_214 |
| ESTHER : Duysen_2011_Toxicol.Appl.Pharmacol_255_214 |
| PubMedSearch : Duysen_2011_Toxicol.Appl.Pharmacol_255_214 |
| PubMedID: 21767560 |
| Title : Exposure to tri-o-cresyl phosphate detected in jet airplane passengers - Liyasova_2011_Toxicol.Appl.Pharmacol_256_337 |
| Author(s) : Liyasova M , Li B , Schopfer LM , Nachon F , Masson P , Furlong CE , Lockridge O |
| Ref : Toxicol Appl Pharmacol , 256 :337 , 2011 |
| Abstract : Liyasova_2011_Toxicol.Appl.Pharmacol_256_337 |
| ESTHER : Liyasova_2011_Toxicol.Appl.Pharmacol_256_337 |
| PubMedSearch : Liyasova_2011_Toxicol.Appl.Pharmacol_256_337 |
| PubMedID: 21723309 |
| Title : Effect of tri-o-cresyl phosphate on cytoskeleton in human neuroblastoma SK-N-SH cell - Chang_2006_Mol.Cell.Biochem_290_145 |
| Author(s) : Chang PA , Wu YJ |
| Ref : Molecular & Cellular Biochemistry , 290 :145 , 2006 |
| Abstract : Chang_2006_Mol.Cell.Biochem_290_145 |
| ESTHER : Chang_2006_Mol.Cell.Biochem_290_145 |
| PubMedSearch : Chang_2006_Mol.Cell.Biochem_290_145 |
| PubMedID: 16909309 |
| Title : Organophosphate induced delayed neuropathy in genetically dissimilar chickens: studies with tri-ortho-cresyl phosphate (TOCP) and trichlorfon - Honorato_2002_Toxicol.Lett_136_143 |
| Author(s) : Honorato de Oliveira G , Moreira V , Ribeiro Goes SP |
| Ref : Toxicol Lett , 136 :143 , 2002 |
| Abstract : Honorato_2002_Toxicol.Lett_136_143 |
| ESTHER : Honorato_2002_Toxicol.Lett_136_143 |
| PubMedSearch : Honorato_2002_Toxicol.Lett_136_143 |
| PubMedID: 12425964 |
| Title : Comparison of neurotoxic effects and potential risks from oral administration or ingestion of tricresyl phosphate and jet engine oil containing tricresyl phosphate - Mackerer_1999_J.Toxicol.Environ.Health_57_293 |
| Author(s) : Mackerer CR , Barth ML , Krueger AJ , Chawla B , Roy TA |
| Ref : J Toxicol Environ Health , 57 :293 , 1999 |
| Abstract : Mackerer_1999_J.Toxicol.Environ.Health_57_293 |
| ESTHER : Mackerer_1999_J.Toxicol.Environ.Health_57_293 |
| PubMedSearch : Mackerer_1999_J.Toxicol.Environ.Health_57_293 |
| PubMedID: 10405186 |
| Title : Inhibition of carboxylesterases in SH-SY5Y human and NB41A3 mouse neuroblastoma cells by organophosphorus esters - Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| Author(s) : Ehrich M , Correll L |
| Ref : J Toxicol Environ Health , 53 :385 , 1998 |
| Abstract : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| ESTHER : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedSearch : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedID: 9515941 |
| Title : The effect of a single oral dose of tri-o-cresyl phosphate on neurotoxic esterase and acetylcholinesterase activities in the central nervous system, erythrocytes and plasma - Barrett_1994_Vet.Hum.Toxicol_36_1 |
| Author(s) : Barrett DS , Oehme FW |
| Ref : Vet Hum Toxicol , 36 :1 , 1994 |
| Abstract : Barrett_1994_Vet.Hum.Toxicol_36_1 |
| ESTHER : Barrett_1994_Vet.Hum.Toxicol_36_1 |
| PubMedSearch : Barrett_1994_Vet.Hum.Toxicol_36_1 |
| PubMedID: 8154093 |
| Title : Clinical manifestations and leukocyte neurotoxic esterase and red blood cell and plasma acetylcholinesterase activities in swine following a single oral dose of tri-o-cresyl phosphate - Barrett_1994_Vet.Hum.Toxicol_36_103 |
| Author(s) : Barrett DS , Oehme FW , Kruckenberg SM , Smith JE |
| Ref : Vet Hum Toxicol , 36 :103 , 1994 |
| Abstract : Barrett_1994_Vet.Hum.Toxicol_36_103 |
| ESTHER : Barrett_1994_Vet.Hum.Toxicol_36_103 |
| PubMedSearch : Barrett_1994_Vet.Hum.Toxicol_36_103 |
| PubMedID: 8197706 |
| Title : Change in hen sciatic nerve calcium after a single oral dose of tri-o-tolyl phosphate - Luttrell_1993_Environ.Res_60_290 |
| Author(s) : Luttrell WE , Olajos EJ , Pleban PA |
| Ref : Environ Research , 60 :290 , 1993 |
| Abstract : Luttrell_1993_Environ.Res_60_290 |
| ESTHER : Luttrell_1993_Environ.Res_60_290 |
| PubMedSearch : Luttrell_1993_Environ.Res_60_290 |
| PubMedID: 8472659 |
| Title : Decrease of phosphofructokinase activity in relation to the pathogenesis of triorthocresyl-phosphate-induced delayed neuropathy - Hernandez_1992_Rev.Esp.Fisiol_48_139 |
| Author(s) : Hernandez AF , Pla A , Villanueva E |
| Ref : Rev Esp Fisiol , 48 :139 , 1992 |
| Abstract : Hernandez_1992_Rev.Esp.Fisiol_48_139 |
| ESTHER : Hernandez_1992_Rev.Esp.Fisiol_48_139 |
| PubMedSearch : Hernandez_1992_Rev.Esp.Fisiol_48_139 |
| PubMedID: 1301629 |
| Title : The interaction of Sertoli and Leydig cells in the testicular toxicity of tri-o-cresyl phosphate - Chapin_1990_Toxicol.Appl.Pharmacol_104_483 |
| Author(s) : Chapin RE , Phelps JL , Somkuti SG , Heindel JJ , Burka LT |
| Ref : Toxicol Appl Pharmacol , 104 :483 , 1990 |
| Abstract : Chapin_1990_Toxicol.Appl.Pharmacol_104_483 |
| ESTHER : Chapin_1990_Toxicol.Appl.Pharmacol_104_483 |
| PubMedSearch : Chapin_1990_Toxicol.Appl.Pharmacol_104_483 |
| PubMedID: 2385838 |
| Title : Effect of acute tri-o-tolyl phosphate exposure on 2', 3'-cyclic nucleotide 3'-phosphohydrolase activity in hen neural tissues - Luttrell_1988_Neurotoxicol_9_539 |
| Author(s) : Luttrell WE , Pleban PA , Olajos EJ |
| Ref : Neurotoxicology , 9 :539 , 1988 |
| Abstract : Luttrell_1988_Neurotoxicol_9_539 |
| ESTHER : Luttrell_1988_Neurotoxicol_9_539 |
| PubMedSearch : Luttrell_1988_Neurotoxicol_9_539 |
| PubMedID: 2849076 |
| Title : Effect of route of administration on the development of organophosphate-induced delayed neurotoxicity in 4-week-old chicks - Olson_1988_J.Toxicol.Environ.Health_23_499 |
| Author(s) : Olson BA , Bursian SJ |
| Ref : J Toxicol Environ Health , 23 :499 , 1988 |
| Abstract : Olson_1988_J.Toxicol.Environ.Health_23_499 |
| ESTHER : Olson_1988_J.Toxicol.Environ.Health_23_499 |
| PubMedSearch : Olson_1988_J.Toxicol.Environ.Health_23_499 |
| PubMedID: 3361617 |
| Title : Low non-neuropathic tri-o-cresyl phosphate (TOCP) doses inhibit neuropathy target esterase near the neuropathic threshold in n-hexane pretreated hens - Pellin_1988_Toxicology_49_99 |
| Author(s) : Pellin MC , Vilanova E , Barril J |
| Ref : Toxicology , 49 :99 , 1988 |
| Abstract : Pellin_1988_Toxicology_49_99 |
| ESTHER : Pellin_1988_Toxicology_49_99 |
| PubMedSearch : Pellin_1988_Toxicology_49_99 |
| PubMedID: 3376128 |
| Title : Toxicity of an acute dose of agent VX and other organophosphorus esters in the chicken - Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| Author(s) : Wilson BW , Henderson JD , Chow E , Schreider J , Goldman M , Culbertson R , Dacre JC |
| Ref : J Toxicol Environ Health , 23 :103 , 1988 |
| Abstract : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| ESTHER : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| PubMedSearch : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| PubMedID: 3336055 |
| Title : Sensitivity to tri-o-cresylphosphate neurotoxicity on n-hexane exposed hens as a model of simultaneous hexacarbon solvent and organophosphorus occupational intoxication - Pellin_1987_Arch.Toxicol_59_311 |
| Author(s) : Pellin M , Vicedo JL , Vilanova E |
| Ref : Archives of Toxicology , 59 :311 , 1987 |
| Abstract : Pellin_1987_Arch.Toxicol_59_311 |
| ESTHER : Pellin_1987_Arch.Toxicol_59_311 |
| PubMedSearch : Pellin_1987_Arch.Toxicol_59_311 |
| PubMedID: 3579594 |
| Title : Acute biological effects of commercial cresyl diphenyl phosphate in rats - Vainiotalo_1987_Toxicology_44_31 |
| Author(s) : Vainiotalo S , Verkkala E , Savolainen H , Nickels J , Zitting A |
| Ref : Toxicology , 44 :31 , 1987 |
| Abstract : Vainiotalo_1987_Toxicology_44_31 |
| ESTHER : Vainiotalo_1987_Toxicology_44_31 |
| PubMedSearch : Vainiotalo_1987_Toxicology_44_31 |
| PubMedID: 3564047 |
| Title : The relationship between neurological damage and neurotoxic esterase inhibition in rats acutely exposed to tri-ortho-cresyl phosphate - Padilla_1985_Toxicol.Appl.Pharmacol_78_78 |
| Author(s) : Padilla S , Veronesi B |
| Ref : Toxicol Appl Pharmacol , 78 :78 , 1985 |
| Abstract : Padilla_1985_Toxicol.Appl.Pharmacol_78_78 |
| ESTHER : Padilla_1985_Toxicol.Appl.Pharmacol_78_78 |
| PubMedSearch : Padilla_1985_Toxicol.Appl.Pharmacol_78_78 |
| PubMedID: 2994253 |
| Title : Characterization of delayed neurotoxicity in the mouse following chronic oral administration of tri-o-cresyl phosphate - Lapadula_1985_Toxicol.Appl.Pharmacol_79_83 |
| Author(s) : Lapadula DM , Patton SE , Campbell GA , Abou-Donia MB |
| Ref : Toxicol Appl Pharmacol , 79 :83 , 1985 |
| Abstract : Lapadula_1985_Toxicol.Appl.Pharmacol_79_83 |
| ESTHER : Lapadula_1985_Toxicol.Appl.Pharmacol_79_83 |
| PubMedSearch : Lapadula_1985_Toxicol.Appl.Pharmacol_79_83 |
| PubMedID: 4049409 |
| Title : [Multiple molecular forms of esterases from grass aphids inhibitory identification and stereospecificity] - Volkova_1983_Biokhimiia_48_1634 |
| Author(s) : Volkova RI , Titova EV |
| Ref : Biokhimiia , 48 :1634 , 1983 |
| Abstract : Volkova_1983_Biokhimiia_48_1634 |
| ESTHER : Volkova_1983_Biokhimiia_48_1634 |
| PubMedSearch : Volkova_1983_Biokhimiia_48_1634 |
| PubMedID: 6639987 |
| Title : Degenerative changes in skeletal muscle of hens with tri-ortho-cresyl phosphate-induced delayed neurotoxicity: altered acetylcholinesterase molecular forms and increased plasma creatine phosphokinase activity - Cisson_1982_Toxicol.Appl.Pharmacol_64_289 |
| Author(s) : Cisson CM , Wilson BW |
| Ref : Toxicol Appl Pharmacol , 64 :289 , 1982 |
| Abstract : Cisson_1982_Toxicol.Appl.Pharmacol_64_289 |
| ESTHER : Cisson_1982_Toxicol.Appl.Pharmacol_64_289 |
| PubMedSearch : Cisson_1982_Toxicol.Appl.Pharmacol_64_289 |
| PubMedID: 7123556 |
| Title : Acute toxicity of tri-ortho-cresyl phosphate in sheep and swine - Wilson_1982_Am.J.Vet.Res_43_1954 |
| Author(s) : Wilson RD , Rowe LD , Lovering SL , Witzel DA |
| Ref : American Journal of Veterinary Research , 43 :1954 , 1982 |
| Abstract : Wilson_1982_Am.J.Vet.Res_43_1954 |
| ESTHER : Wilson_1982_Am.J.Vet.Res_43_1954 |
| PubMedSearch : Wilson_1982_Am.J.Vet.Res_43_1954 |
| PubMedID: 7181194 |
| Title : The influence of 2-\/o-cresyl\/-4 H-1 : 3 : 2-benzodioxa-phosphorin-2-oxide (CBDP) on organophosphate poisoning and its therapy - Boskovic_1979_Arch.Toxicol_42_207 |
| Author(s) : Boskovic B |
| Ref : Archives of Toxicology , 42 :207 , 1979 |
| Abstract : Boskovic_1979_Arch.Toxicol_42_207 |
| ESTHER : Boskovic_1979_Arch.Toxicol_42_207 |
| PubMedSearch : Boskovic_1979_Arch.Toxicol_42_207 |
| PubMedID: 475595 |
| Title : Effects of tricresylphosphate on esterase activity of rat serum and tissues - Manno_1979_Br.J.Ind.Med_36_153 |
| Author(s) : Manno M , Rigoni F , Bartolucci GB , Bianchi M , Mazzotta M |
| Ref : British Journal of Industrial Medicine , 36 :153 , 1979 |
| Abstract : Manno_1979_Br.J.Ind.Med_36_153 |
| ESTHER : Manno_1979_Br.J.Ind.Med_36_153 |
| PubMedSearch : Manno_1979_Br.J.Ind.Med_36_153 |
| PubMedID: 465377 |
| Title : Evaluation of cytotoxic responses caused by selected organophosphorus esters in chick sympathetic ganglia cultures - Obersteiner_1978_Can.J.Comp.Med_42_80 |
| Author(s) : Obersteiner EJ , Sharma RP |
| Ref : Can J Comp Med , 42 :80 , 1978 |
| Abstract : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| ESTHER : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedSearch : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedID: 565668 |
| Title : Synaptosomal adenosine triphosphatase (ATPase) inhibition by organophosphates - Brown_1976_Experientia_32_1540 |
| Author(s) : Brown HR , Sharma RP |
| Ref : Experientia , 32 :1540 , 1976 |
| Abstract : Brown_1976_Experientia_32_1540 |
| ESTHER : Brown_1976_Experientia_32_1540 |
| PubMedSearch : Brown_1976_Experientia_32_1540 |
| PubMedID: 139320 |
| Title : Changes in the central nervous system in the cat as the result of tri-o-cresylphosphate poisoning - |
| Author(s) : Cavanagh JB , Patangia GN |
| Ref : Brain , 88 :165 , 1965 |
| PubMedID: 14280272 |
| Title : The effect of tri-o-cresyl phosphate intoxication on phospholipid synthesis in cat spinal cord - |
| Author(s) : Taylor JD |
| Ref : Canadian Journal of Physiology & Pharmacology , 43 :715 , 1965 |
| PubMedID: |
| Title : Effects of certain metabolically active drugs and oximes on tri-o-cresyl phosphate toxicity - |
| Author(s) : Bleiberg MJ , Johnson H |
| Ref : Toxicology & Applied Pharmacology , 7 :227 , 1965 |
| PubMedID: 14298014 |
| Title : Peripheral nerve changes in orthocresylphosphate poisoning, in the cat - |
| Author(s) : Cavanagh JB |
| Ref : Journal of Pathology & Bacteriology , 87 :365 , 1964 |
| PubMedID: 14137697 |
| Title : Biological activity of a tri-o-cresyl phosphate metabolite - Casida_1961_Nature_191_1396 |
| Author(s) : Casida JE , Eto M , Baron RL |
| Ref : Nature , 191 :1396 , 1961 |
| Abstract : Casida_1961_Nature_191_1396 |
| ESTHER : Casida_1961_Nature_191_1396 |
| PubMedSearch : Casida_1961_Nature_191_1396 |
| PubMedID: |
| Title : Comparison of the functional effects of dyflos, tri-o-cresyl phosphate and tri-p-ethylphenyl phosphate in chickens - Cavanagh_1961_Br.J.Pharmacol.Chemother_17_21 |
| Author(s) : Cavanagh JB , Davies DR , Holland P , Lancaster M |
| Ref : British Journal of Pharmacology and Chemotherapy , 17 :21 , 1961 |
| Abstract : Cavanagh_1961_Br.J.Pharmacol.Chemother_17_21 |
| ESTHER : Cavanagh_1961_Br.J.Pharmacol.Chemother_17_21 |
| PubMedSearch : Cavanagh_1961_Br.J.Pharmacol.Chemother_17_21 |
| PubMedID: 13691738 |
| Title : Outbreak of paralysis in Morocco due to ortho-cresyl phosphate poisoning - |
| Author(s) : Smith HV , Spalding JMK |
| Ref : Lancet , 11 :1019 , 1959 |
| PubMedID: |
| Title : Tricresyl phosphates and cholinesterase - |
| Author(s) : Aldridge WN |
| Ref : Biochemical Journal , 56 :185 , 1954 |
| PubMedID: 13140171 |
| Title : The toxic effects of triortho-cresyl phosphate on the nervous system\; an experimental study in hens - |
| Author(s) : Cavanagh JB |
| Ref : Journal of Neurology Neurosurg Psychiatry , 17 :163 , 1954 |
| PubMedID: 13192490 |
| Title : Observations on the specificity of the inhibition of cholinesterases by tri-orthocresyl phosphate - |
| Author(s) : Earl CJ , Thompson RHS , Webster GR |
| Ref : British Journal of Pharmacology and Chemotherapy , 8 :110 , 1953 |
| PubMedID: |
| Title : Cholinesterase levels in the nervous system in tri-ortho-cresyl phosphate poisoning - |
| Author(s) : Earl CJ , Thompson RHS |
| Ref : British Journal of Pharmacology and Chemotherapy , 7 :685 , 1952 |
| PubMedID: 13019039 |
| Title : Specificity of cholinesterase inhibition by tri-o-cresyl phosphate - |
| Author(s) : Hottinger A , Bloch H |
| Ref : Helv Chim Acta , 26 :142 , 1943 |
| PubMedID: |
| Title : The systemic nervous activity of triorthocresyl phosphate (Jamaica Ginger Palsy) - |
| Author(s) : Ahring CD |
| Ref : Brain , 65 :34 , 1942 |
| PubMedID: |