Taxifolin
2,3-dihydro derivative of quercetin. Effective free radical scavenger. Taxifolin had an IC50 value of 30.1 nM and a Ki value of 16.7 +/- 3.9 nM non-competitive and not specific: inhibits carbonic anhydrase isoenzymes I and II similarily. Dihydroquercetin is proposed for the treatment of oxidative stress in severe acute respiratory syndrome COVID-19
General
Type : Natural,Flavonoid
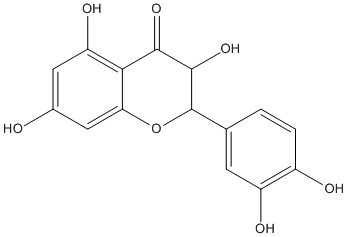
Chemical_Nomenclature : 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one
Canonical SMILES : C1=CC(=C(C=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
InChI : InChI=1S\/C15H12O7\/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6\/h1-5,14-19,21H
InChIKey : CXQWRCVTCMQVQX-UHFFFAOYSA-N
Other name(s) : dihydroquercetin,(+\/-)-Taxifolin,NSC2801,3,3',4',5,7-Pentahydroxyflavanone,CHEBI:38747,2,3-Dihydroquercetin,98006-93-0,(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one,4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-,AK146105
MW : 304.25
Formula : C15H12O7
CAS_number : 215257-15-1
PubChem : 471
UniChem : CXQWRCVTCMQVQX-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (4)
| Title : 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo - Gunesch_2020_Redox.Biol_29_101378 |
| Author(s) : Gunesch S , Hoffmann M , Kiermeier C , Fischer W , Pinto AFM , Maurice T , Maher P , Decker M |
| Ref : Redox Biol , 29 :101378 , 2020 |
| Abstract : Gunesch_2020_Redox.Biol_29_101378 |
| ESTHER : Gunesch_2020_Redox.Biol_29_101378 |
| PubMedSearch : Gunesch_2020_Redox.Biol_29_101378 |
| PubMedID: 31926632 |
| Title : Screening and identification of secondary metabolites in the bark of Bauhinia variegata to treat Alzheimer's disease by using molecular docking and molecular dynamics simulations - Khare_2020_J.Biomol.Struct.Dyn__1 |
| Author(s) : Khare N , Maheshwari SK , Jha AK |
| Ref : J Biomol Struct Dyn , :1 , 2020 |
| Abstract : Khare_2020_J.Biomol.Struct.Dyn__1 |
| ESTHER : Khare_2020_J.Biomol.Struct.Dyn__1 |
| PubMedSearch : Khare_2020_J.Biomol.Struct.Dyn__1 |
| PubMedID: 32720564 |
| Title : Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease 2019 (COVID-19) and its complications - Mironova_2020_Eur.Rev.Med.Pharmacol.Sci_24_8585 |
| Author(s) : Mironova GD , Belosludtseva NV , Ananyan MA |
| Ref : Eur Rev Med Pharmacol Sci , 24 :8585 , 2020 |
| Abstract : Mironova_2020_Eur.Rev.Med.Pharmacol.Sci_24_8585 |
| ESTHER : Mironova_2020_Eur.Rev.Med.Pharmacol.Sci_24_8585 |
| PubMedSearch : Mironova_2020_Eur.Rev.Med.Pharmacol.Sci_24_8585 |
| PubMedID: 32894566 |
| Title : Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin - Gocer_2016_J.Enzyme.Inhib.Med.Chem_31_441 |
| Author(s) : Gocer H , Topal F , Topal M , Kucuk M , Teke D , Gulcin I , Alwasel SH , Supuran CT |
| Ref : J Enzyme Inhib Med Chem , 31 :441 , 2016 |
| Abstract : Gocer_2016_J.Enzyme.Inhib.Med.Chem_31_441 |
| ESTHER : Gocer_2016_J.Enzyme.Inhib.Med.Chem_31_441 |
| PubMedSearch : Gocer_2016_J.Enzyme.Inhib.Med.Chem_31_441 |
| PubMedID: 25893707 |