TAK-147
Transition state analogue
General
Type : Piperidine,Derivative of Donepezil,Transition state analogue,Benzazepin
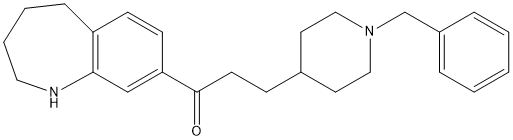
Chemical_Nomenclature : 3-(1-benzylpiperidin-4-yl)-1-(2,3,4,5-tetrahydro-1H-1-benzazepin-8-yl)propan-1-one
Canonical SMILES : C1CCNC2=C(C1)C=CC(=C2)C(=O)CCC3CCN(CC3)CC4=CC=CC=C4.C(=CC(=O)O)C(=O)O || C1CCNC2=C(C1)C=CC(=C2)C(=O)CCC3CCN(CC3)CC4=CC=CC=C4
InChI : nChI=1S\/C25H32N2O.C4H4O4\/c28-25(23-11-10-22-8-4-5-15-26-24(22)18-23)12-9-20-13-16-27(17-14-20)19-21-6-2-1-3-7-21\;5-3(6)1-2-4(7)8\/h1-3,6-7,10-11,18,20,26H,4-5,8-9,12-17,19H2\;1-2H,(H,5,6)(H,7,8)\/b\;2-1+ || InChI=1S\/C25H32N2O\/c28-25(23-11-10-22-8-4-5-15-26-24(22)18-23)12-9-20-13-16-27(17-14-20)19-21-6-2-1-3-7-21\/h1-3,6-7,10-11,18,20,26H,4-5,8-9,12-17,19H2
InChIKey : CWEHWZPCDBRUNO-WLHGVMLRSA-N || PMBLXLOXUGVTGB-UHFFFAOYSA-N
Other name(s) : zanapezil,Zanapezil fumarate,Zanepezil fumarate,C13424,UNII-0A0800O89N,CHEMBL75013,SCHEMBL678642,CHEBI:188916
MW : 492.607
Formula : C29H36N2O5 || C25H32N2O
CAS_number : 263248-42-6 || 142852-50-4
UniChem : CWEHWZPCDBRUNO-WLHGVMLRSA-N || PMBLXLOXUGVTGB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (8)
| Title : Effect of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1H-1-benzazepin-8-yl)-1- propanone fumarate, a novel acetylcholinesterase inhibitor, on spatial cognitive impairment induced by chronic cerebral hypoperfusion in rats - Xu_2002_Neurosci.Lett_331_33 |
| Author(s) : Xu AJ , Chen Z , Yanai K , Huang YW , Wei EQ |
| Ref : Neuroscience Letters , 331 :33 , 2002 |
| Abstract : Xu_2002_Neurosci.Lett_331_33 |
| ESTHER : Xu_2002_Neurosci.Lett_331_33 |
| PubMedSearch : Xu_2002_Neurosci.Lett_331_33 |
| PubMedID: 12359317 |
| Title : TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons - Kato_1999_Neurosci.Lett_260_5 |
| Author(s) : Kato K , Hayako H , Ishihara Y , Marui S , Iwane M , Miyamoto M |
| Ref : Neuroscience Letters , 260 :5 , 1999 |
| Abstract : Kato_1999_Neurosci.Lett_260_5 |
| ESTHER : Kato_1999_Neurosci.Lett_260_5 |
| PubMedSearch : Kato_1999_Neurosci.Lett_260_5 |
| PubMedID: 10027686 |
| Title : Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats - Kosasa_1999_Eur.J.Pharmacol_380_101 |
| Author(s) : Kosasa T , Kuriya Y , Matsui K , Yamanishi Y |
| Ref : European Journal of Pharmacology , 380 :101 , 1999 |
| Abstract : Kosasa_1999_Eur.J.Pharmacol_380_101 |
| ESTHER : Kosasa_1999_Eur.J.Pharmacol_380_101 |
| PubMedSearch : Kosasa_1999_Eur.J.Pharmacol_380_101 |
| PubMedID: 10513568 |
| Title : Effect of donepezil hydrochloride (E2020) on extracellular acetylcholine concentration in the cerebral cortex of rats - Kosasa_1999_Jpn.J.Pharmacol_81_216 |
| Author(s) : Kosasa T , Kuriya Y , Yamanishi Y |
| Ref : Japanese Journal of Pharmacology , 81 :216 , 1999 |
| Abstract : Kosasa_1999_Jpn.J.Pharmacol_81_216 |
| ESTHER : Kosasa_1999_Jpn.J.Pharmacol_81_216 |
| PubMedSearch : Kosasa_1999_Jpn.J.Pharmacol_81_216 |
| PubMedID: 10591480 |
| Title : Neurochemical effects of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1H-1-b enzazepin-8-yl)-1-propanone fumarate (TAK-147), a novel acetylcholinesterase inhibitor, in rats - Hirai_1997_J.Pharmacol.Exp.Ther_280_1261 |
| Author(s) : Hirai K , Kato K , Nakayama T , Hayako H , Ishihara Y , Goto G , Miyamoto M |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 280 :1261 , 1997 |
| Abstract : Hirai_1997_J.Pharmacol.Exp.Ther_280_1261 |
| ESTHER : Hirai_1997_J.Pharmacol.Exp.Ther_280_1261 |
| PubMedSearch : Hirai_1997_J.Pharmacol.Exp.Ther_280_1261 |
| PubMedID: 9067312 |
| Title : Effects of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1 - H-1-benzazepin-8-yl)-1-propanone fumarate (TAK-147), a novel acetylcholinesterase inhibitor, on impaired learning and memory in animal models - Miyamoto_1996_J.Pharmacol.Exp.Ther_277_1292 |
| Author(s) : Miyamoto M , Takahashi H , Kato K , Hirai K , Ishihara Y , Goto G |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 277 :1292 , 1996 |
| Abstract : Miyamoto_1996_J.Pharmacol.Exp.Ther_277_1292 |
| ESTHER : Miyamoto_1996_J.Pharmacol.Exp.Ther_277_1292 |
| PubMedSearch : Miyamoto_1996_J.Pharmacol.Exp.Ther_277_1292 |
| PubMedID: 8667190 |
| Title : Effect of TAK-147, a novel AChE inhibitor, on cerebral energy metabolism - Nakayama_1996_Neurobiol.Aging_17_849 |
| Author(s) : Nakayama T , Takahashi H , Miyamoto M , Goto G , Nagai Y |
| Ref : Neurobiology of Aging , 17 :849 , 1996 |
| Abstract : Nakayama_1996_Neurobiol.Aging_17_849 |
| ESTHER : Nakayama_1996_Neurobiol.Aging_17_849 |
| PubMedSearch : Nakayama_1996_Neurobiol.Aging_17_849 |
| PubMedID: 9363795 |
| Title : Central cholinergic agents. 6. Synthesis and evaluation of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1H-1-benzazepi n-8-yl)-1-propanones and their analogs as central selective acetylcholinesterase inhibitors - Ishihara_1994_J.Med.Chem_37_2292 |
| Author(s) : Ishihara Y , Hirai K , Miyamoto M , Goto G |
| Ref : Journal of Medicinal Chemistry , 37 :2292 , 1994 |
| Abstract : Ishihara_1994_J.Med.Chem_37_2292 |
| ESTHER : Ishihara_1994_J.Med.Chem_37_2292 |
| PubMedSearch : Ishihara_1994_J.Med.Chem_37_2292 |
| PubMedID: 8057278 |