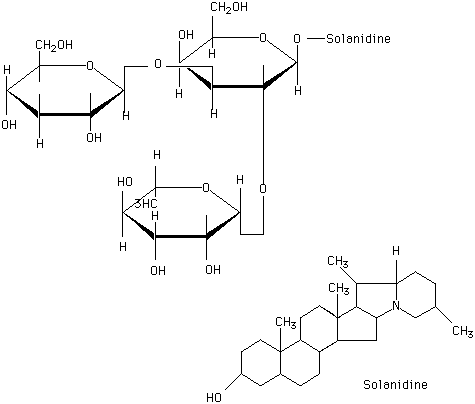
Solanine
A trisaccharide,consisting of glucose,galactose and rhamnose linked to solanidine(glucose 2rhamnose for chaconine) purified from potato sprouts. CAS 20562-03-02 C45H73NO14 alpha-chaconine ; CAS 20562-02-1 ,C45H73NO15 Solanine
General
Type : Natural,Alkaloid,Carbohydrate,Glycoside
Chemical_Nomenclature : beta-D-Glucopyranoside, (3beta)-solanid-5-en-3-yl O-6-deoxy-alpha-L-mannopyranosyl-(1-2)-O-(6-deoxy-alpha-L-mannopyranosyl-(1-4))
Canonical SMILES : CC1CCC2C(C3C(N2C1)CC4C3(CCC5C4CC=C6C5(CCC(C6)OC7C(C(C(C(O7)CO)O)OC8C(C(C(C(O8)CO)O)O)O)OC9C(C(C(C(O9)C)O)O)O)C)C)C
InChI : InChI=1S\/C45H73NO15\/c1-19-6-9-27-20(2)31-28(46(27)16-19)15-26-24-8-7-22-14-23(10-12-44(22,4)25(24)11-13-45(26,31)5)57-43-40(61-41-37(54)35(52)32(49)21(3)56-41)39(34(51)30(18-48)59-43)60-42-38(55)36(53)33(50)29(17-47)58-42\/h7,19-21,23-43,47-55H,6,8-18H2,1-5H3
InChIKey : ZGVSETXHNHBTRK-UHFFFAOYSA-N
Other name(s) : alpha-Solanine,alpha-Solanin,AC1L67M3,SCHEMBL2380177,CHEMBL1975187,NSC96019,NSC-96019
MW : 868.1
Formula : C45H73NO15
CAS_number : 20562-02-1
PubChem : 262500. alpha-Solanine is found in alcoholic beverages. alpha-Solanine is an alkaloid from potato (Solanum tuberosum) and very many other Solanum species (Solanaceae). Responsible for the teratogenicity of sprouting potatoes, 262500
UniChem : ZGVSETXHNHBTRK-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (12)
| Title : The Impact of Steroidal Glycoalkaloids on the Physiology of Phytophthora infestans, the Causative Agent of Potato Late Blight - Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| Author(s) : Dahlin P , Muller MC , Ekengren S , McKee LS , Bulone V |
| Ref : Mol Plant Microbe Interact , 30 :531 , 2017 |
| Abstract : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| ESTHER : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| PubMedSearch : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| PubMedID: 28510502 |
| Title : Detection of glycoalkaloids using disposable biosensors based on genetically modified enzymes - Espinoza_2014_Anal.Biochem_457_85 |
| Author(s) : Espinoza MA , Istamboulie G , Chira A , Noguer T , Stoytcheva M , Marty JL |
| Ref : Analytical Biochemistry , 457 :85 , 2014 |
| Abstract : Espinoza_2014_Anal.Biochem_457_85 |
| ESTHER : Espinoza_2014_Anal.Biochem_457_85 |
| PubMedSearch : Espinoza_2014_Anal.Biochem_457_85 |
| PubMedID: 24747413 |
| Title : Acute toxicity of high doses of the glycoalkaloids, alpha-solanine and alpha-chaconine, in the Syrian Golden hamster - Langkilde_2008_J.Agric.Food.Chem_56_8753 |
| Author(s) : Langkilde S , Schroder M , Stewart D , Meyer O , Conner S , Davies H , Poulsen M |
| Ref : Journal of Agricultural and Food Chemistry , 56 :8753 , 2008 |
| Abstract : Langkilde_2008_J.Agric.Food.Chem_56_8753 |
| ESTHER : Langkilde_2008_J.Agric.Food.Chem_56_8753 |
| PubMedSearch : Langkilde_2008_J.Agric.Food.Chem_56_8753 |
| PubMedID: 18710251 |
| Title : A novel enzyme biosensor for steroidal glycoalkaloids detection based on pH-sensitive field effect transistors - Korpan_2002_Bioelectrochemistry_55_9 |
| Author(s) : Korpan YI , Volotovsky VV , Martelet C , Jaffrezic-Renault N , Nazarenko EA , El'skaya AV , Soldatkin AP |
| Ref : Bioelectrochemistry , 55 :9 , 2002 |
| Abstract : Korpan_2002_Bioelectrochemistry_55_9 |
| ESTHER : Korpan_2002_Bioelectrochemistry_55_9 |
| PubMedSearch : Korpan_2002_Bioelectrochemistry_55_9 |
| PubMedID: 11786329 |
| Title : Cholinesterase inhibition by potato glycoalkaloids slows mivacurium metabolism - McGehee_2000_Anesthesiology_93_510 |
| Author(s) : McGehee DS , Krasowski MD , Fung DL , Wilson B , Gronert GA , Moss J |
| Ref : Anesthesiology , 93 :510 , 2000 |
| Abstract : McGehee_2000_Anesthesiology_93_510 |
| ESTHER : McGehee_2000_Anesthesiology_93_510 |
| PubMedSearch : McGehee_2000_Anesthesiology_93_510 |
| PubMedID: 10910502 |
| Title : Normal and atypical butyrylcholinesterases in placental development, function, and malfunction - Sternfeld_1997_Cell.Mol.Neurobiol_17_315 |
| Author(s) : Sternfeld M , Rachmilewitz J , Loewenstein-Lichtenstein Y , Andres C , Timberg R , Ben-Ari S , Glick C , Soreq H , Zakut H |
| Ref : Cellular Molecular Neurobiology , 17 :315 , 1997 |
| Abstract : Sternfeld_1997_Cell.Mol.Neurobiol_17_315 |
| ESTHER : Sternfeld_1997_Cell.Mol.Neurobiol_17_315 |
| PubMedSearch : Sternfeld_1997_Cell.Mol.Neurobiol_17_315 |
| PubMedID: 9187488 |
| Title : Overlapping drug interaction sites of human butyrylcholinesterase dissected by site-directed mutagenesis - Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| Author(s) : Loewenstein-Lichtenstein Y , Glick D , Gluzman N , Sternfeld M , Zakut H , Soreq H |
| Ref : Molecular Pharmacology , 50 :1423 , 1996 |
| Abstract : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| ESTHER : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedSearch : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedID: 8967962 |
| Gene_locus related to this paper: human-BCHE |
| Title : A Point Mutation of Acetylcholinesterase Associated with Azinphosmethyl Resistance and Reduced Fitness in Colorado Potato Beetle - Zhu_1996_Pestic.Biochem.Physiol_55_100 |
| Author(s) : Zhu KY , Lee SH , Clark JM |
| Ref : Pestic Biochem Physiol , 55 :100 , 1996 |
| Abstract : Zhu_1996_Pestic.Biochem.Physiol_55_100 |
| ESTHER : Zhu_1996_Pestic.Biochem.Physiol_55_100 |
| PubMedSearch : Zhu_1996_Pestic.Biochem.Physiol_55_100 |
| PubMedID: 8980034 |
| Gene_locus related to this paper: lepde-ACHE |
| Title : Inhibition of human plasma and serum butyrylcholinesterase (EC 3.1.1.8) by alpha-chaconine and alpha-solanine - Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| Author(s) : Nigg HN , Ramos LE , Graham EM , Sterling J , Brown S , Cornell JA |
| Ref : Fundamental & Applied Toxicology , 33 :272 , 1996 |
| Abstract : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| ESTHER : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| PubMedSearch : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| PubMedID: 8921346 |
| Title : Tissue distribution of human acetylcholinesterase and butyrylcholinesterase messenger RNA - Jbilo_1994_Toxicon_32_1445 |
| Author(s) : Jbilo O , Bartels CF , Chatonnet A , Toutant JP , Lockridge O |
| Ref : Toxicon , 32 :1445 , 1994 |
| Abstract : Jbilo_1994_Toxicon_32_1445 |
| ESTHER : Jbilo_1994_Toxicon_32_1445 |
| PubMedSearch : Jbilo_1994_Toxicon_32_1445 |
| PubMedID: 7886701 |
| Title : Inhibition of insect acetylcholinesterase by the potato glucoalkaloid-chaconine - Wierenga_1993_Nat.Toxins_1_96 |
| Author(s) : Wierenga JM , Hollingworth RM |
| Ref : Nat Toxins , 1 :96 , 1993 |
| Abstract : Wierenga_1993_Nat.Toxins_1_96 |
| ESTHER : Wierenga_1993_Nat.Toxins_1_96 |
| PubMedSearch : Wierenga_1993_Nat.Toxins_1_96 |
| PubMedID: 1344914 |
| Title : Differential inhibition of the serum cholinesterase phenotypes by solanine and solanidine - |
| Author(s) : Harris H , Whittaker M |
| Ref : Annals of Human Genetics , 26 :73 , 1962 |
| PubMedID: 13904837 |