Saxagliptin
Saxagliptin Anhydrous is the anhydrous form of saxagliptin, a potent, selective and competitive, cyanopyrrolidine-based, orally bioavailable inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity. Saxagliptin is metabolized into an, although less potent, active mono-hydroxy metabolite
General
Type : Adamantyl,Drug,Cyanide,Pyrrolidine,Gliptin,Azabicyclo
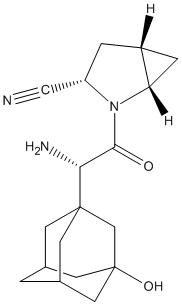
Chemical_Nomenclature : (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-1-adamantyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile
Canonical SMILES : C1C2CC2N(C1C#N)C(=O)C(C34CC5CC(C3)CC(C5)(C4)O)N
InChI : InChI=1S\/C18H25N3O2\/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17\/h10-15,23H,1-7,9,20H2\/t10?,11?,12-,13+,14+,15-,17?,18?\/m1\/s1
InChIKey : QGJUIPDUBHWZPV-SGTAVMJGSA-N
Other name(s) : Onglyza,BJM,BMS-477118,CHEMBL385517,CHEBI:71272
MW : 315.41
Formula : C18H25N3O2
CAS_number : 361442-04-8
PubChem : 11243969
UniChem : QGJUIPDUBHWZPV-SGTAVMJGSA-N
IUPHAR : 6316
Wikipedia : Saxagliptin
Target
Families : Saxagliptin ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 3BJM Crystal structure of human DPP-IV in complex with (1S,3S, 5S)-2-[(2S)-2-amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1- yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile (CAS)OR BMS-477118
Protein : human-DPP4
References (14)
| Title : Covalent Inhibition Mechanism of Antidiabetic DrugsVildagliptin vs Saxagliptin - Wang_2019_ACS.Catalysis_9_2292 |
| Author(s) : Wang YH , Zhang F , Diao H , Wu R |
| Ref : ACS Catal , 9 :2292 , 2019 |
| Abstract : Wang_2019_ACS.Catalysis_9_2292 |
| ESTHER : Wang_2019_ACS.Catalysis_9_2292 |
| PubMedSearch : Wang_2019_ACS.Catalysis_9_2292 |
| PubMedID: |
| Title : Pharmacokinetic, pharmacodynamic and clinical evaluation of saxagliptin in type 2 diabetes - Anderson_2016_Expert.Opin.Drug.Metab.Toxicol_12_467 |
| Author(s) : Anderson R , Hayes J , Stephens JW |
| Ref : Expert Opin Drug Metab Toxicol , 12 :467 , 2016 |
| Abstract : Anderson_2016_Expert.Opin.Drug.Metab.Toxicol_12_467 |
| ESTHER : Anderson_2016_Expert.Opin.Drug.Metab.Toxicol_12_467 |
| PubMedSearch : Anderson_2016_Expert.Opin.Drug.Metab.Toxicol_12_467 |
| PubMedID: 26878666 |
| Title : Glucose-independent renoprotective mechanisms of the tissue dipeptidyl peptidase-4 inhibitor, saxagliptin, in Dahl salt-sensitive hypertensive rats - Uchii_2016_Eur.J.Pharmacol_783_56 |
| Author(s) : Uchii M , Kimoto N , Sakai M , Kitayama T , Kunori S |
| Ref : European Journal of Pharmacology , 783 :56 , 2016 |
| Abstract : Uchii_2016_Eur.J.Pharmacol_783_56 |
| ESTHER : Uchii_2016_Eur.J.Pharmacol_783_56 |
| PubMedSearch : Uchii_2016_Eur.J.Pharmacol_783_56 |
| PubMedID: 27063445 |
| Title : Saxagliptin: a dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes mellitus - Neumiller_2010_Am.J.Health.Syst.Pharm_67_1515 |
| Author(s) : Neumiller JJ , Campbell RK |
| Ref : Am J Health Syst Pharm , 67 :1515 , 2010 |
| Abstract : Neumiller_2010_Am.J.Health.Syst.Pharm_67_1515 |
| ESTHER : Neumiller_2010_Am.J.Health.Syst.Pharm_67_1515 |
| PubMedSearch : Neumiller_2010_Am.J.Health.Syst.Pharm_67_1515 |
| PubMedID: 20811029 |
| Title : Saxagliptin: the evidence for its place in the treatment of type 2 diabetes mellitus - Kulasa_2010_Core.Evid_5_23 |
| Author(s) : Kulasa K , Edelman S |
| Ref : Core Evid , 5 :23 , 2010 |
| Abstract : Kulasa_2010_Core.Evid_5_23 |
| ESTHER : Kulasa_2010_Core.Evid_5_23 |
| PubMedSearch : Kulasa_2010_Core.Evid_5_23 |
| PubMedID: 21042540 |
| Title : Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for type 2 diabetes - Lam_2010_Cardiol.Rev_18_213 |
| Author(s) : Lam S , Saad M |
| Ref : Cardiol Rev , 18 :213 , 2010 |
| Abstract : Lam_2010_Cardiol.Rev_18_213 |
| ESTHER : Lam_2010_Cardiol.Rev_18_213 |
| PubMedSearch : Lam_2010_Cardiol.Rev_18_213 |
| PubMedID: 20539105 |
| Title : Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes - Gupta_2009_Curr.Drug.Targets_10_71 |
| Author(s) : Gupta R , Walunj SS , Tokala RK , Parsa KV , Singh SK , Pal M |
| Ref : Curr Drug Targets , 10 :71 , 2009 |
| Abstract : Gupta_2009_Curr.Drug.Targets_10_71 |
| ESTHER : Gupta_2009_Curr.Drug.Targets_10_71 |
| PubMedSearch : Gupta_2009_Curr.Drug.Targets_10_71 |
| PubMedID: 19149538 |
| Title : Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin--diabetes control and potential adverse events - Ahren_2009_Best.Pract.Res.Clin.Endocrinol.Metab_23_487 |
| Author(s) : Ahren B |
| Ref : Best Pract Res Clinical Endocrinology Metab , 23 :487 , 2009 |
| Abstract : Ahren_2009_Best.Pract.Res.Clin.Endocrinol.Metab_23_487 |
| ESTHER : Ahren_2009_Best.Pract.Res.Clin.Endocrinol.Metab_23_487 |
| PubMedSearch : Ahren_2009_Best.Pract.Res.Clin.Endocrinol.Metab_23_487 |
| PubMedID: 19748066 |
| Title : Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes - Deacon_2009_Adv.Ther_26_488 |
| Author(s) : Deacon CF , Holst JJ |
| Ref : Adv Ther , 26 :488 , 2009 |
| Abstract : Deacon_2009_Adv.Ther_26_488 |
| ESTHER : Deacon_2009_Adv.Ther_26_488 |
| PubMedSearch : Deacon_2009_Adv.Ther_26_488 |
| PubMedID: 19444391 |
| Title : Pharmacokinetics of the dipeptidyl peptidase 4 inhibitor saxagliptin in rats, dogs, and monkeys and clinical projections - Fura_2009_Drug.Metab.Dispos_37_1164 |
| Author(s) : Fura A , Khanna A , Vyas V , Koplowitz B , Chang SY , Caporuscio C , Boulton DW , Christopher LJ , Chadwick KD , Hamann LG , Humphreys WG , Kirby M |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 37 :1164 , 2009 |
| Abstract : Fura_2009_Drug.Metab.Dispos_37_1164 |
| ESTHER : Fura_2009_Drug.Metab.Dispos_37_1164 |
| PubMedSearch : Fura_2009_Drug.Metab.Dispos_37_1164 |
| PubMedID: 19251818 |
| Title : Saxagliptin, a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes - Gallwitz_2008_IDrugs_11_906 |
| Author(s) : Gallwitz B |
| Ref : IDrugs , 11 :906 , 2008 |
| Abstract : Gallwitz_2008_IDrugs_11_906 |
| ESTHER : Gallwitz_2008_IDrugs_11_906 |
| PubMedSearch : Gallwitz_2008_IDrugs_11_906 |
| PubMedID: 19051153 |
| Title : Involvement of DPP-IV catalytic residues in enzyme-saxagliptin complex formation - Metzler_2008_Protein.Sci_17_240 |
| Author(s) : Metzler WJ , Yanchunas J , Weigelt C , Kish K , Klei HE , Xie D , Zhang Y , Corbett M , Tamura JK , He B , Hamann LG , Kirby MS , Marcinkeviciene J |
| Ref : Protein Science , 17 :240 , 2008 |
| Abstract : Metzler_2008_Protein.Sci_17_240 |
| ESTHER : Metzler_2008_Protein.Sci_17_240 |
| PubMedSearch : Metzler_2008_Protein.Sci_17_240 |
| PubMedID: 18227430 |
| Gene_locus related to this paper: human-DPP4 |
| Title : Biocatalytic ammonolysis of (5S)-4,5-dihydro-1H-pyrrole-1,5-dicarboxylic acid, 1-(1,1-dimethylethyl)-5-ethyl ester: preparation of an intermediate to the dipeptidyl peptidase IV inhibitor Saxagliptin - Gill_2006_Bioorg.Med.Chem.Lett_16_705 |
| Author(s) : Gill I , Patel R |
| Ref : Bioorganic & Medicinal Chemistry Lett , 16 :705 , 2006 |
| Abstract : Gill_2006_Bioorg.Med.Chem.Lett_16_705 |
| ESTHER : Gill_2006_Bioorg.Med.Chem.Lett_16_705 |
| PubMedSearch : Gill_2006_Bioorg.Med.Chem.Lett_16_705 |
| PubMedID: 16257208 |
| Gene_locus related to this paper: canar-LipB |
| Title : Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes - Augeri_2005_J.Med.Chem_48_5025 |
| Author(s) : Augeri DJ , Robl JA , Betebenner DA , Magnin DR , Khanna A , Robertson JG , Wang A , Simpkins LM , Taunk P , Huang Q , Han SP , Abboa-Offei B , Cap M , Xin L , Tao L , Tozzo E , Welzel GE , Egan DM , Marcinkeviciene J , Chang SY , Biller SA , Kirby MS , Parker RA , Hamann LG |
| Ref : Journal of Medicinal Chemistry , 48 :5025 , 2005 |
| Abstract : Augeri_2005_J.Med.Chem_48_5025 |
| ESTHER : Augeri_2005_J.Med.Chem_48_5025 |
| PubMedSearch : Augeri_2005_J.Med.Chem_48_5025 |
| PubMedID: 16033281 |