Sanguinarine
Sanguinarine is a benzophenanthridine alkaloid, an alkaloid antibiotic and a botanical anti-fungal agent. || IC50 ACHE 1.22 muM (1.10-1.34) BChE 7.08 muM (4.27-10.2)
General
Type : Natural,Alkaloid,Not A\/B H target,Benzodioxo,Phenanthridinium
Chemical_Nomenclature : 24-methyl-5,7,18,20-tetraoxa-24-azoniahexacyclo[11.11.0.02,10.04,8.014,22.017,21]tetracosa-1(24),2,4(8),9,11,13,15,17(21),22-nonaene
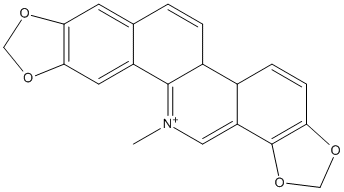
Canonical SMILES : C[N+]1=C2C(=C3C=CC4=C(C3=C1)OCO4)C=CC5=CC6=C(C=C52)OCO6
InChI : InChI=1S\/C20H14NO4\/c1-21-8-15-12(4-5-16-20(15)25-10-22-16)13-3-2-11-6-17-18(24-9-23-17)7-14(11)19(13)21\/h2-8H,9-10H2,1H3\/q+1
InChIKey : INVGWHRKADIJHF-UHFFFAOYSA-N
Other name(s) : Sanguinarin,Pseudochelerythrine,sangvinarin,CHEMBL417799,CHEBI:17183,ZINC706
MW : 332.3
Formula : C20H14NO4+
CAS_number : 2447-54-3
PubChem : 5154
UniChem : INVGWHRKADIJHF-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (5)
| Title : Application of HPLC-DAD for In Vitro Investigation of Acetylcholinesterase Inhibition Activity of Selected Isoquinoline Alkaloids from Sanguinaria canadensis Extracts - Tuzimski_2021_Molecules_26_ |
| Author(s) : Tuzimski T , Petruczynik A |
| Ref : Molecules , 26 : , 2021 |
| Abstract : Tuzimski_2021_Molecules_26_ |
| ESTHER : Tuzimski_2021_Molecules_26_ |
| PubMedSearch : Tuzimski_2021_Molecules_26_ |
| PubMedID: 33466254 |
| Title : Simple analogues of natural product chelerythrine: Discovery of a novel anticholinesterase 2-phenylisoquinolin-2-ium scaffold with excellent potency against acetylcholinesterase - Zhou_2020_Eur.J.Med.Chem_200_112415 |
| Author(s) : Zhou B , Li H , Cui Z , Li D , Geng H , Gao J , Zhou L |
| Ref : Eur Journal of Medicinal Chemistry , 200 :112415 , 2020 |
| Abstract : Zhou_2020_Eur.J.Med.Chem_200_112415 |
| ESTHER : Zhou_2020_Eur.J.Med.Chem_200_112415 |
| PubMedSearch : Zhou_2020_Eur.J.Med.Chem_200_112415 |
| PubMedID: 32454229 |
| Title : Bioactive isoquinoline alkaloids from Corydalis saxicola - Huang_2012_Planta.Med_78_65 |
| Author(s) : Huang QQ , Bi JL , Sun QY , Yang FM , Wang YH , Tang GH , Zhao FW , Wang H , Xu JJ , Kennelly EJ , Long CL , Yin GF |
| Ref : Planta Med , 78 :65 , 2012 |
| Abstract : Huang_2012_Planta.Med_78_65 |
| ESTHER : Huang_2012_Planta.Med_78_65 |
| PubMedSearch : Huang_2012_Planta.Med_78_65 |
| PubMedID: 21858757 |
| Title : [The inhibition enzymatic hydrolysis of acetylthiocholine by acetylcholinesterase using principal alkaloids isolated from celandine and macleya and their derivatives] - Kuznetsova_2001_Tsitologiia_43_1046 |
| Author(s) : Kuznetsova LP , Nikol'skaia EB , Sochilina EE , Faddeeva MD |
| Ref : Tsitologiia , 43 :1046 , 2001 |
| Abstract : Kuznetsova_2001_Tsitologiia_43_1046 |
| ESTHER : Kuznetsova_2001_Tsitologiia_43_1046 |
| PubMedSearch : Kuznetsova_2001_Tsitologiia_43_1046 |
| PubMedID: 11840780 |
| Title : Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores - Schmeller_1997_Phytochemistry_44_257 |
| Author(s) : Schmeller T , Latz-Bruning B , Wink M |
| Ref : Phytochemistry , 44 :257 , 1997 |
| Abstract : Schmeller_1997_Phytochemistry_44_257 |
| ESTHER : Schmeller_1997_Phytochemistry_44_257 |
| PubMedSearch : Schmeller_1997_Phytochemistry_44_257 |
| PubMedID: 9004542 |