SCHEMBL19358888
dual sEH/FAAH inhibitor human-EPHX2 IC50=3nM mouse-hyes IC50=2.1 nM human FAAH IC50=24nM mouse FAAH IC50=510nM
General
Type : Urea derivative,Benzoate,FAAH inhibitor,Multitarget
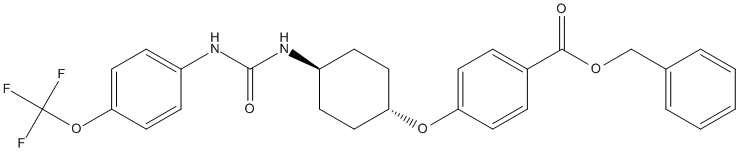
Chemical_Nomenclature : benzyl 4-[4-[[4-(trifluoromethoxy)phenyl]carbamoylamino]cyclohexyl]oxybenzoate
Canonical SMILES : C1CC(CCC1NC(=O)NC2=CC=C(C=C2)OC(F)(F)F)OC3=CC=C(C=C3)C(=O)OCC4=CC=CC=C4
InChI : InChI=1S\/C28H27F3N2O5\/c29-28(30,31)38-25-16-10-22(11-17-25)33-27(35)32-21-8-14-24(15-9-21)37-23-12-6-20(7-13-23)26(34)36-18-19-4-2-1-3-5-19\/h1-7,10-13,16-17,21,24H,8-9,14-15,18H2,(H2,32,33,35)
InChIKey : IEOQZCMTPHDRLT-UHFFFAOYSA-N
Other name(s) : compound 24
MW : 528.52
Formula : C28H27F3N2O5
CAS_number :
PubChem : 130431481
UniChem : IEOQZCMTPHDRLT-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : SCHEMBL19358888 ligand of proteins in family: Epoxide_hydrolase
Stucture :
Protein : human-EPHX2 || mouse-hyes
References (1)
| Title : Identification and optimization of soluble epoxide hydrolase inhibitors with dual potency towards fatty acid amide hydrolase - Kodani_2018_Bioorg.Med.Chem.Lett_28_762 |
| Author(s) : Kodani SD , Bhakta S , Hwang SH , Pakhomova S , Newcomer ME , Morisseau C , Hammock BD |
| Ref : Bioorganic & Medicinal Chemistry Lett , 28 :762 , 2018 |
| Abstract : Kodani_2018_Bioorg.Med.Chem.Lett_28_762 |
| ESTHER : Kodani_2018_Bioorg.Med.Chem.Lett_28_762 |
| PubMedSearch : Kodani_2018_Bioorg.Med.Chem.Lett_28_762 |
| PubMedID: 29366648 |
| Gene_locus related to this paper: human-EPHX2 |