Rilapladib
Rilapladib is the third genomics-derived small molecule drug arising from the Human Genome Sciences-GlaxoSmithKline collaboration to enter clinical development. It is a lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor. Lp-PLA2 is an enzyme associated with the formation of atherosclerotic plaques.
General
Type : Trifluoro,Piperidine,Sulfur Compound
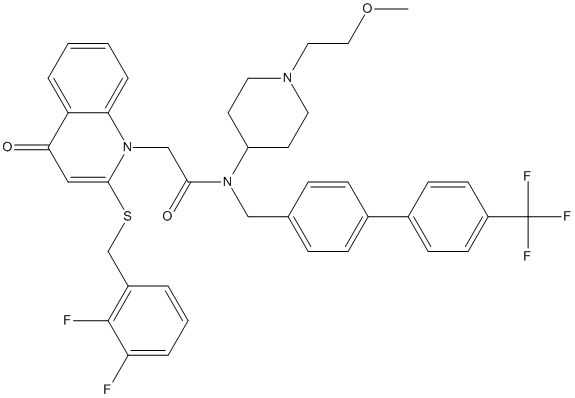
Chemical_Nomenclature : 2-[2-[(2,3-difluorophenyl)methylsulfanyl]-4-oxoquinolin-1-yl]-N-[1-(2-methoxyethyl)piperidin-4-yl]-N-[[4-[4-(trifluoromethyl)phenyl]phenyl]methyl]acetamide
Canonical SMILES : COCCN1CCC(CC1)N(CC2=CC=C(C=C2)C3=CC=C(C=C3)C(F)(F)F)C(=O)CN4C5=CC=CC=C5C(=O)C=C4SCC6=C(C(=CC=C6)F)F
InChI : InChI=1S\/C40H38F5N3O3S\/c1-51-22-21-46-19-17-32(18-20-46)47(24-27-9-11-28(12-10-27)29-13-15-31(16-14-29)40(43,44)45)37(50)25-48-35-8-3-2-6-33(35)36(49)23-38(48)52-26-30-5-4-7-34(41)39(30)42\/h2-16,23,32H,17-22,24-26H2,1H3
InChIKey : NNBGCSGCRSCFEA-UHFFFAOYSA-N
Other name(s) : UNII-O14CWE893Z,Rilapladib (USAN),SB 659032,SB659032,D05728,DB05119
MW : 735.80
Formula : C40H38F5N3O3S
CAS_number : 412950-08-4
PubChem : 9918381
UniChem : NNBGCSGCRSCFEA-UHFFFAOYSA-N
IUPHAR : 7376
Wikipedia :
Target
Families : Rilapladib ligand of proteins in family: PAF-Acetylhydrolase
Stucture :
Protein : human-PLA2G7
References (6)
| Title : A 24-week study to evaluate the effect of rilapladib on cognition and cerebrospinal fluid biomarkers of Alzheimer's disease - Maher-Edwards_2015_Alzheimers.Dement.(N.Y)_1_131 |
| Author(s) : Maher-Edwards G , De'Ath J , Barnett C , Lavrov A , Lockhart A |
| Ref : Alzheimers Dement (N Y) , 1 :131 , 2015 |
| Abstract : Maher-Edwards_2015_Alzheimers.Dement.(N.Y)_1_131 |
| ESTHER : Maher-Edwards_2015_Alzheimers.Dement.(N.Y)_1_131 |
| PubMedSearch : Maher-Edwards_2015_Alzheimers.Dement.(N.Y)_1_131 |
| PubMedID: 29854933 |
| Title : Lipoprotein-associated phospholipase A2 and risk of dementia in the Cardiovascular Health Study - Fitzpatrick_2014_Atherosclerosis_235_384 |
| Author(s) : Fitzpatrick AL , Irizarry MC , Cushman M , Jenny NS , Chi GC , Koro C |
| Ref : Atherosclerosis , 235 :384 , 2014 |
| Abstract : Fitzpatrick_2014_Atherosclerosis_235_384 |
| ESTHER : Fitzpatrick_2014_Atherosclerosis_235_384 |
| PubMedSearch : Fitzpatrick_2014_Atherosclerosis_235_384 |
| PubMedID: 24929287 |
| Title : Platelet aggregation unchanged by lipoprotein-associated phospholipase A inhibition: results from an in vitro study and two randomized phase I trials - Shaddinger_2014_PLoS.One_9_e83094 |
| Author(s) : Shaddinger BC , Xu Y , Roger JH , Macphee CH , Handel M , Baidoo CA , Magee M , Lepore JJ , Sprecher DL |
| Ref : PLoS ONE , 9 :e83094 , 2014 |
| Abstract : Shaddinger_2014_PLoS.One_9_e83094 |
| ESTHER : Shaddinger_2014_PLoS.One_9_e83094 |
| PubMedSearch : Shaddinger_2014_PLoS.One_9_e83094 |
| PubMedID: 24475026 |
| Title : Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging - |
| Author(s) : Tawakol A , Singh P , Rudd JH , Soffer J , Cai G , Vucic E , Brannan SP , Tarka EA , Shaddinger BC , Sarov-Blat L , Matthews P , Subramanian S , Farkouh M , Fayad ZA |
| Ref : J Am Coll Cardiol , 63 :86 , 2014 |
| PubMedID: 23973698 |
| Title : Plasma lipoprotein-associated phospholipase A2 activity in Alzheimer's disease, amnestic mild cognitive impairment, and cognitively healthy elderly subjects: a cross-sectional study - Davidson_2012_Alzheimers.Res.Ther_4_51 |
| Author(s) : Davidson JE , Lockhart A , Amos L , Stirnadel-Farrant HA , Mooser V , Sollberger M , Regeniter A , Monsch AU , Irizarry MC |
| Ref : Alzheimers Res Ther , 4 :51 , 2012 |
| Abstract : Davidson_2012_Alzheimers.Res.Ther_4_51 |
| ESTHER : Davidson_2012_Alzheimers.Res.Ther_4_51 |
| PubMedSearch : Davidson_2012_Alzheimers.Res.Ther_4_51 |
| PubMedID: 23217243 |
| Title : Lipoprotein-associated phospholipase A(2) and atherosclerosis - Wilensky_2009_Curr.Opin.Lipidol_20_415 |
| Author(s) : Wilensky RL , Macphee CH |
| Ref : Curr Opin Lipidol , 20 :415 , 2009 |
| Abstract : Wilensky_2009_Curr.Opin.Lipidol_20_415 |
| ESTHER : Wilensky_2009_Curr.Opin.Lipidol_20_415 |
| PubMedSearch : Wilensky_2009_Curr.Opin.Lipidol_20_415 |
| PubMedID: 19667981 |