Pyridostigmine
Pyridostigmine is a cholinesterase inhibitor with a slightly longer duration of action than Neostigmine. It is used in the treatment of myasthenia gravis and to reverse the actions of muscle relaxants.
General
Type : Carbamate,Drug,Pyridine
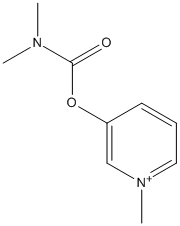
Chemical_Nomenclature : 3-[[(Dimethylamino)-carbonyl]oxy]-1-methylpyridinium bromide
Canonical SMILES : C[N+]1=CC=CC(=C1)OC(=O)N(C)C
InChI : InChI=1S\/C9H13N2O2\/c1-10(2)9(12)13-8-5-4-6-11(3)7-8\/h4-7H,1-3H3\/q+1
InChIKey : RVOLLAQWKVFTGE-UHFFFAOYSA-N
Other name(s) : Pyridostigminum,Regonol,Mestinon-SR,UNII-19QM69HH21,Pyridostigmine Bromine
MW : 261.12
Formula : C9H13BrN2O2
CAS_number : 101-26-8
PubChem : 4991
UniChem : RVOLLAQWKVFTGE-UHFFFAOYSA-N
IUPHAR : 8994
Wikipedia : Pyridostigmine
Target
Families : Pyridostigmine ligand of proteins in family: BCHE || ACHE
Stucture :
Protein : human-ACHE || human-BCHE
References (216)
| Title : Rare Case of Iatrogenic Myocardial Infarction Induced by Use of Pyridostigmine - Niazi_2020_Cureus_12_e9849 |
| Author(s) : Niazi M , Iqbal QZ , Zia Z , Sattar SBA , Lafferty J |
| Ref : Cureus , 12 :e9849 , 2020 |
| Abstract : Niazi_2020_Cureus_12_e9849 |
| ESTHER : Niazi_2020_Cureus_12_e9849 |
| PubMedSearch : Niazi_2020_Cureus_12_e9849 |
| PubMedID: 32953356 |
| Title : Pyridostigmine Impairs Pulmonary Function in Asthmatic Subjects: Reanalysis of Results From an Observational Study - |
| Author(s) : Hansen MRH , Schlunssen V |
| Ref : Mil Med , : , 2020 |
| PubMedID: 32207521 |
| Title : Neuromuscular blockade reversal with sugammadex versus pyridostigmine\/glycopyrrolate in laparoscopic cholecystectomy: a randomized trial of effects on postoperative gastrointestinal motility - An_2020_Korean.J.Anesthesiol_73_137 |
| Author(s) : An J , Noh H , Kim E , Lee J , Woo K , Kim H |
| Ref : Korean J Anesthesiol , 73 :137 , 2020 |
| Abstract : An_2020_Korean.J.Anesthesiol_73_137 |
| ESTHER : An_2020_Korean.J.Anesthesiol_73_137 |
| PubMedSearch : An_2020_Korean.J.Anesthesiol_73_137 |
| PubMedID: 31636242 |
| Title : A parallel-group, multicenter randomized, double-blinded, placebo-controlled, phase 2\/3, clinical trial to test the efficacy of pyridostigmine bromide at low doses to reduce mortality or invasive mechanical ventilation in adults with severe SARS-CoV-2 infection: the Pyridostigmine In Severe COvid-19 (PISCO) trial protocol - Fragoso-Saavedra_2020_BMC.Infect.Dis_20_765 |
| Author(s) : Fragoso-Saavedra S , Iruegas-Nunez DA , Quintero-Villegas A , Garcia-Gonzalez HB , Nunez I , Carbajal-Morelos SL , Audelo-Cruz BM , Arias-Martinez S , Caro-Vega Y , Calva JJ , Luqueno-Martinez V , Gonzalez-Duarte A , Crabtree-Ramirez B , Crispin JC , Sierra-Madero J , Belaunzaran-Zamudio PF , Valdes-Ferrer SI |
| Ref : BMC Infect Dis , 20 :765 , 2020 |
| Abstract : Fragoso-Saavedra_2020_BMC.Infect.Dis_20_765 |
| ESTHER : Fragoso-Saavedra_2020_BMC.Infect.Dis_20_765 |
| PubMedSearch : Fragoso-Saavedra_2020_BMC.Infect.Dis_20_765 |
| PubMedID: 33066761 |
| Title : Acetylcholinesterase Inhibitor Pyridostigmine Bromide Attenuates Gut Pathology and Bacterial Dysbiosis in a Murine Model of Ulcerative Colitis - Singh_2020_Dig.Dis.Sci_65_141 |
| Author(s) : Singh SP , Chand HS , Banerjee S , Agarwal H , Raizada V , Roy S , Sopori M |
| Ref : Digestive Diseases & Sciences , 65 :141 , 2020 |
| Abstract : Singh_2020_Dig.Dis.Sci_65_141 |
| ESTHER : Singh_2020_Dig.Dis.Sci_65_141 |
| PubMedSearch : Singh_2020_Dig.Dis.Sci_65_141 |
| PubMedID: 31643033 |
| Title : Interactions between pyridostigmine bromide and stress on glutamatergic neurochemistry: Insights from a rat model of Gulf War Illness - Macht_2020_Neurobiol.Stress_12_100210 |
| Author(s) : Macht VA , Woodruff JL , Burzynski HE , Grillo CA , Reagan LP , Fadel JR |
| Ref : Neurobiol Stress , 12 :100210 , 2020 |
| Abstract : Macht_2020_Neurobiol.Stress_12_100210 |
| ESTHER : Macht_2020_Neurobiol.Stress_12_100210 |
| PubMedSearch : Macht_2020_Neurobiol.Stress_12_100210 |
| PubMedID: 32258255 |
| Title : The Val16Ala-SOD2 polymorphism affects cyto-genotoxicity of pyridostigmine bromide on human peripheral blood mononuclear cells - Azzolin_2019_Toxicol.In.Vitro_60_237 |
| Author(s) : Azzolin VF , Barbisan F , Teixeira CF , Pillar D , Mastella MH , Duarte T , Turra BO , Ribeiro EE , Duarte M , da Cruz IBM |
| Ref : Toxicol In Vitro , 60 :237 , 2019 |
| Abstract : Azzolin_2019_Toxicol.In.Vitro_60_237 |
| ESTHER : Azzolin_2019_Toxicol.In.Vitro_60_237 |
| PubMedSearch : Azzolin_2019_Toxicol.In.Vitro_60_237 |
| PubMedID: 31175926 |
| Title : beta2-Adrenergic receptor agonists ameliorate the adverse effect of long-term pyridostigmine on neuromuscular junction structure - Vanhaesebrouck_2019_Brain_142_3713 |
| Author(s) : Vanhaesebrouck AE , Webster R , Maxwell S , Rodriguez Cruz PM , Cossins J , Wickens J , Liu WW , Cetin H , Cheung J , Ramjattan H , Palace J , Beeson D |
| Ref : Brain , 142 :3713 , 2019 |
| Abstract : Vanhaesebrouck_2019_Brain_142_3713 |
| ESTHER : Vanhaesebrouck_2019_Brain_142_3713 |
| PubMedSearch : Vanhaesebrouck_2019_Brain_142_3713 |
| PubMedID: 31633155 |
| Title : Cholinergic Stimulation by Pyridostigmine Bromide Before Myocardial Infarction Prevent Cardiac and Autonomic Dysfunction - Barboza_2019_Sci.Rep_9_2481 |
| Author(s) : Barboza CA , Fukushima AR , Carrozzi N , Machi JF , Dourado PMM , Mostarda CT , Irigoyen MC , Nathanson L , Morris M , Caperuto EC , Rodrigues B |
| Ref : Sci Rep , 9 :2481 , 2019 |
| Abstract : Barboza_2019_Sci.Rep_9_2481 |
| ESTHER : Barboza_2019_Sci.Rep_9_2481 |
| PubMedSearch : Barboza_2019_Sci.Rep_9_2481 |
| PubMedID: 30792425 |
| Title : The effect of pyridostigmine on small intestinal bacterial overgrowth (SIBO) and plasma inflammatory biomarkers in HIV-associated autonomic neuropathies - Robinson-Papp_2019_J.Neurovirol_25_551 |
| Author(s) : Robinson-Papp J , Nmashie A , Pedowitz E , George MC , Sharma S , Murray J , Benn EKT , Lawrence SA , Machac J , Heiba S , Kim-Schulze S , Navis A , Roland BC , Morgello S |
| Ref : J Neurovirol , 25 :551 , 2019 |
| Abstract : Robinson-Papp_2019_J.Neurovirol_25_551 |
| ESTHER : Robinson-Papp_2019_J.Neurovirol_25_551 |
| PubMedSearch : Robinson-Papp_2019_J.Neurovirol_25_551 |
| PubMedID: 31098925 |
| Title : Pyridostigmine bromide and stress interact to impact immune function, cholinergic neurochemistry and behavior in a rat model of Gulf War Illness - Macht_2019_Brain.Behav.Immun_80_384 |
| Author(s) : Macht VA , Woodruff JL , Maissy ES , Grillo CA , Wilson MA , Fadel JR , Reagan LP |
| Ref : Brain Behavior & Immunity , 80 :384 , 2019 |
| Abstract : Macht_2019_Brain.Behav.Immun_80_384 |
| ESTHER : Macht_2019_Brain.Behav.Immun_80_384 |
| PubMedSearch : Macht_2019_Brain.Behav.Immun_80_384 |
| PubMedID: 30953774 |
| Title : Pretreatment with pyridostigmine bromide has no effect on seizure behavior or 24 hour survival in the rat model of acute diisopropylfluorophosphate intoxication - Bruun_2019_Neurotoxicol_73_81 |
| Author(s) : Bruun DA , Guignet M , Harvey DJ , Lein PJ |
| Ref : Neurotoxicology , 73 :81 , 2019 |
| Abstract : Bruun_2019_Neurotoxicol_73_81 |
| ESTHER : Bruun_2019_Neurotoxicol_73_81 |
| PubMedSearch : Bruun_2019_Neurotoxicol_73_81 |
| PubMedID: 30853371 |
| Title : Synergistic Pressor Effect of Atomoxetine and Pyridostigmine in Patients With Neurogenic Orthostatic Hypotension - Okamoto_2019_Hypertension_73_235 |
| Author(s) : Okamoto LE , Shibao CA , Gamboa A , Diedrich A , Raj SR , Black BK , Robertson D , Biaggioni I |
| Ref : Hypertension , 73 :235 , 2019 |
| Abstract : Okamoto_2019_Hypertension_73_235 |
| ESTHER : Okamoto_2019_Hypertension_73_235 |
| PubMedSearch : Okamoto_2019_Hypertension_73_235 |
| PubMedID: 30571543 |
| Title : Pyridostigmine Suicidal Attempt in a Myasthenia Gravis Patient - Malabaey_2019_Am.J.Case.Rep_20_1418 |
| Author(s) : Malabaey MAT , Al-Saud AA , Alaska YA , Almas A , Malik A |
| Ref : Am J Case Rep , 20 :1418 , 2019 |
| Abstract : Malabaey_2019_Am.J.Case.Rep_20_1418 |
| ESTHER : Malabaey_2019_Am.J.Case.Rep_20_1418 |
| PubMedSearch : Malabaey_2019_Am.J.Case.Rep_20_1418 |
| PubMedID: 31554781 |
| Title : Pyridostigmine in Pediatric Intestinal Pseudo-obstruction: Case Report of a 2-year Old Girl and Literature Review - Nardo_2019_J.Neurogastroenterol.Motil_25_508 |
| Author(s) : Nardo GD , Viscogliosi F , Esposito F , Stanghellini V , Villa MP , Parisi P , Morlando A , Cal G , Giorgio R |
| Ref : J Neurogastroenterol Motil , 25 :508 , 2019 |
| Abstract : Nardo_2019_J.Neurogastroenterol.Motil_25_508 |
| ESTHER : Nardo_2019_J.Neurogastroenterol.Motil_25_508 |
| PubMedSearch : Nardo_2019_J.Neurogastroenterol.Motil_25_508 |
| PubMedID: 31587541 |
| Title : Pyridostigmine alleviates cardiac dysfunction via improving mitochondrial cristae shape in a mouse model of metabolic syndrome - Xue_2019_Free.Radic.Biol.Med_134_119 |
| Author(s) : Xue RQ , Yu XJ , Zhao M , Xu M , Wu Q , Cui YL , Yang S , Li DL , Zang WJ |
| Ref : Free Radic Biol Med , 134 :119 , 2019 |
| Abstract : Xue_2019_Free.Radic.Biol.Med_134_119 |
| ESTHER : Xue_2019_Free.Radic.Biol.Med_134_119 |
| PubMedSearch : Xue_2019_Free.Radic.Biol.Med_134_119 |
| PubMedID: 30633969 |
| Title : Application of Pyridostigmine in Pediatric Gastrointestinal Motility Disorders: A Case Series - Manini_2018_Paediatr.Drugs_20_173 |
| Author(s) : Manini ML , Camilleri M , Grothe R , Di Lorenzo C |
| Ref : Paediatr Drugs , 20 :173 , 2018 |
| Abstract : Manini_2018_Paediatr.Drugs_20_173 |
| ESTHER : Manini_2018_Paediatr.Drugs_20_173 |
| PubMedSearch : Manini_2018_Paediatr.Drugs_20_173 |
| PubMedID: 29243034 |
| Title : Gulf War agents pyridostigmine bromide and permethrin cause hypersensitive nociception that is restored after vagus nerve stimulation - Nizamutdinov_2018_Neurotoxicol_69_93 |
| Author(s) : Nizamutdinov D , Mukherjee S , Deng C , Stauss HM , Shapiro LA |
| Ref : Neurotoxicology , 69 :93 , 2018 |
| Abstract : Nizamutdinov_2018_Neurotoxicol_69_93 |
| ESTHER : Nizamutdinov_2018_Neurotoxicol_69_93 |
| PubMedSearch : Nizamutdinov_2018_Neurotoxicol_69_93 |
| PubMedID: 30273628 |
| Title : Pyridostigmine for the Reversal of Severe Adverse Reactions to Botulinum Toxin in Children - Boerner_2018_J.Pediatr_194_241 |
| Author(s) : Boerner RM , Young DL , Gnagi SH , White DR , Halstead LA |
| Ref : J Pediatr , 194 :241 , 2018 |
| Abstract : Boerner_2018_J.Pediatr_194_241 |
| ESTHER : Boerner_2018_J.Pediatr_194_241 |
| PubMedSearch : Boerner_2018_J.Pediatr_194_241 |
| PubMedID: 29275924 |
| Title : Pyridostigmine protects against cardiomyopathy associated with adipose tissue browning and improvement of vagal activity in high-fat diet rats - Lu_2018_Biochim.Biophys.Acta_1864_1037 |
| Author(s) : Lu Y , Wu Q , Liu LZ , Yu XJ , Liu JJ , Li MX , Zang WJ |
| Ref : Biochimica & Biophysica Acta , 1864 :1037 , 2018 |
| Abstract : Lu_2018_Biochim.Biophys.Acta_1864_1037 |
| ESTHER : Lu_2018_Biochim.Biophys.Acta_1864_1037 |
| PubMedSearch : Lu_2018_Biochim.Biophys.Acta_1864_1037 |
| PubMedID: 29309922 |
| Title : Protocol for a phase II, monocentre, double-blind, placebo-controlled, cross-over trial to assess efficacy of pyridostigmine in patients with spinal muscular atrophy types 2-4 (SPACE trial) - Stam_2018_BMJ.Open_8_e019932 |
| Author(s) : Stam M , Wadman RI , Wijngaarde CA , Bartels B , Asselman FL , Otto LAM , Goedee HS , Habets LE , de Groot JF , Schoenmakers M , Cuppen I , van den Berg LH , van der Pol WL |
| Ref : BMJ Open , 8 :e019932 , 2018 |
| Abstract : Stam_2018_BMJ.Open_8_e019932 |
| ESTHER : Stam_2018_BMJ.Open_8_e019932 |
| PubMedSearch : Stam_2018_BMJ.Open_8_e019932 |
| PubMedID: 30061431 |
| Title : Pathophysiology in a model of Gulf War Illness: Contributions of pyridostigmine bromide and stress - Macht_2018_Psychoneuroendocrinology_96_195 |
| Author(s) : Macht VA , Woodruff JL , Grillo CA , Wood CS , Wilson MA , Reagan LP |
| Ref : Psychoneuroendocrinology , 96 :195 , 2018 |
| Abstract : Macht_2018_Psychoneuroendocrinology_96_195 |
| ESTHER : Macht_2018_Psychoneuroendocrinology_96_195 |
| PubMedSearch : Macht_2018_Psychoneuroendocrinology_96_195 |
| PubMedID: 30041099 |
| Title : Pyridostigmine for the treatment of gastrointestinal symptoms in systemic sclerosis - Ahuja_2018_Semin.Arthritis.Rheum_48_111 |
| Author(s) : Ahuja NK , Mische L , Clarke JO , Wigley FM , McMahan ZH |
| Ref : Semin Arthritis Rheum , 48 :111 , 2018 |
| Abstract : Ahuja_2018_Semin.Arthritis.Rheum_48_111 |
| ESTHER : Ahuja_2018_Semin.Arthritis.Rheum_48_111 |
| PubMedSearch : Ahuja_2018_Semin.Arthritis.Rheum_48_111 |
| PubMedID: 29397195 |
| Title : Subacute pyridostigmine exposure increases heart rate recovery and cardiac parasympathetic tone in rats - Bharadwaj_2017_Clin.Exp.Pharmacol.Physiol_44_872 |
| Author(s) : Bharadwaj M , Pope C , Davis M , Katz S , Cook C , Maxwell L |
| Ref : Clinical & Experimental Pharmacology & Physiology , 44 :872 , 2017 |
| Abstract : Bharadwaj_2017_Clin.Exp.Pharmacol.Physiol_44_872 |
| ESTHER : Bharadwaj_2017_Clin.Exp.Pharmacol.Physiol_44_872 |
| PubMedSearch : Bharadwaj_2017_Clin.Exp.Pharmacol.Physiol_44_872 |
| PubMedID: 28440910 |
| Title : Effect of pyridostigmine on in vivo and in vitro respiratory muscle of mdx mice - Amancio_2017_Respir.Physiol.Neurobiol_243_107 |
| Author(s) : Amancio GCS , Grabe-Guimaraes A , Haikel D , Moreau J , Barcellos NMS , Lacampagne A , Matecki S , Cazorla O |
| Ref : Respir Physiol Neurobiol , 243 :107 , 2017 |
| Abstract : Amancio_2017_Respir.Physiol.Neurobiol_243_107 |
| ESTHER : Amancio_2017_Respir.Physiol.Neurobiol_243_107 |
| PubMedSearch : Amancio_2017_Respir.Physiol.Neurobiol_243_107 |
| PubMedID: 28624507 |
| Title : Long-term administration of pyridostigmine attenuates pressure overload-induced cardiac hypertrophy by inhibiting calcineurin signalling - Lu_2017_J.Cell.Mol.Med_21_2106 |
| Author(s) : Lu Y , Zhao M , Liu JJ , He X , Yu XJ , Liu LZ , Sun L , Chen LN , Zang WJ |
| Ref : J Cell Mol Med , 21 :2106 , 2017 |
| Abstract : Lu_2017_J.Cell.Mol.Med_21_2106 |
| ESTHER : Lu_2017_J.Cell.Mol.Med_21_2106 |
| PubMedSearch : Lu_2017_J.Cell.Mol.Med_21_2106 |
| PubMedID: 28296184 |
| Title : The Evaluation of Benefit of Newly Prepared Reversible Inhibitors of Acetylcholinesterase and Commonly Used Pyridostigmine as Pharmacological Pretreatment of Soman-Poisoned Mice - Kassa_2017_Acta.Medica.(Hradec.Kralove)_60_37 |
| Author(s) : Kassa J , Korabecny J , Nepovimova E |
| Ref : Acta Medica (Hradec Kralove) , 60 :37 , 2017 |
| Abstract : Kassa_2017_Acta.Medica.(Hradec.Kralove)_60_37 |
| ESTHER : Kassa_2017_Acta.Medica.(Hradec.Kralove)_60_37 |
| PubMedSearch : Kassa_2017_Acta.Medica.(Hradec.Kralove)_60_37 |
| PubMedID: 28418831 |
| Title : Effects of Pyridostigmine bromide on SH-SY5Y cells: An in vitro neuroblastoma neurotoxicity model - Azzolin_2017_Mutat.Res_823_1 |
| Author(s) : Azzolin VF , Barbisan F , Lenz LS , Teixeira CF , Fortuna M , Duarte T , Duarte M , da Cruz IBM |
| Ref : Mutat Res , 823 :1 , 2017 |
| Abstract : Azzolin_2017_Mutat.Res_823_1 |
| ESTHER : Azzolin_2017_Mutat.Res_823_1 |
| PubMedSearch : Azzolin_2017_Mutat.Res_823_1 |
| PubMedID: 28985942 |
| Title : Pyridostigmine Induced Prolonged Asystole in a Patient with Myasthenia Gravis Successfully Treated with Hyoscyamine - Khan_2017_Case.Rep.Cardiol_2017_6956298 |
| Author(s) : Khan MS , Tiwari A , Khan Z , Sharma H , Taleb M , Hammersley J |
| Ref : Case Rep Cardiol , 2017 :6956298 , 2017 |
| Abstract : Khan_2017_Case.Rep.Cardiol_2017_6956298 |
| ESTHER : Khan_2017_Case.Rep.Cardiol_2017_6956298 |
| PubMedSearch : Khan_2017_Case.Rep.Cardiol_2017_6956298 |
| PubMedID: 28589042 |
| Title : Novel potent pyridoxine-based inhibitors of AChE and BChE, structural analogs of pyridostigmine, with improved in vivo safety profile - Strelnik_2016_Bioorg.Med.Chem.Lett_26_4092 |
| Author(s) : Strelnik AD , Petukhov AS , Zueva IV , Zobov VV , Petrov KA , Nikolsky EE , Balakin KV , Bachurin SO , Shtyrlin YG |
| Ref : Bioorganic & Medicinal Chemistry Lett , 26 :4092 , 2016 |
| Abstract : Strelnik_2016_Bioorg.Med.Chem.Lett_26_4092 |
| ESTHER : Strelnik_2016_Bioorg.Med.Chem.Lett_26_4092 |
| PubMedSearch : Strelnik_2016_Bioorg.Med.Chem.Lett_26_4092 |
| PubMedID: 27377327 |
| Title : Leukocytoclastic Vasculitis Secondary to Pyridostigmine (Mestinon): Report of a Possible First Case - Singh_2016_Perm.J_21_ |
| Author(s) : Singh G , Hodgson T , Clarke DE |
| Ref : Perm J , 21 : , 2016 |
| Abstract : Singh_2016_Perm.J_21_ |
| ESTHER : Singh_2016_Perm.J_21_ |
| PubMedSearch : Singh_2016_Perm.J_21_ |
| PubMedID: 28080955 |
| Title : Comparison of pyridostigmine and bisacodyl in the treatment of refractory chronic constipation - Soufi-Afshar_2016_Caspian.J.Intern.Med_7_19 |
| Author(s) : Soufi-Afshar I , Moghadamnia A , Bijani A , Kazemi S , Shokri-Shirvani J |
| Ref : Caspian J Intern Med , 7 :19 , 2016 |
| Abstract : Soufi-Afshar_2016_Caspian.J.Intern.Med_7_19 |
| ESTHER : Soufi-Afshar_2016_Caspian.J.Intern.Med_7_19 |
| PubMedSearch : Soufi-Afshar_2016_Caspian.J.Intern.Med_7_19 |
| PubMedID: 26958328 |
| Title : Cholinergic stimulation with pyridostigmine protects myocardial infarcted rats against ischemic-induced arrhythmias and preserves connexin43 protein - Santos-Almeida_2015_Am.J.Physiol.Heart.Circ.Physiol_308_H101 |
| Author(s) : Santos-Almeida FM , Girao H , da Silva CA , Salgado HC , Fazan R, Jr. |
| Ref : American Journal of Physiology Heart Circ Physiol , 308 :H101 , 2015 |
| Abstract : Santos-Almeida_2015_Am.J.Physiol.Heart.Circ.Physiol_308_H101 |
| ESTHER : Santos-Almeida_2015_Am.J.Physiol.Heart.Circ.Physiol_308_H101 |
| PubMedSearch : Santos-Almeida_2015_Am.J.Physiol.Heart.Circ.Physiol_308_H101 |
| PubMedID: 25416192 |
| Title : Pyridostigmine enhances atrial tachyarrhythmias in aging spontaneously hypertensive rats - Sayin_2015_Clin.Exp.Pharmacol.Physiol_42_1084 |
| Author(s) : Sayin H , Scridon A , Orea V , Chapuis B , Chevalier P , Barres C , Julien C |
| Ref : Clinical & Experimental Pharmacology & Physiology , 42 :1084 , 2015 |
| Abstract : Sayin_2015_Clin.Exp.Pharmacol.Physiol_42_1084 |
| ESTHER : Sayin_2015_Clin.Exp.Pharmacol.Physiol_42_1084 |
| PubMedSearch : Sayin_2015_Clin.Exp.Pharmacol.Physiol_42_1084 |
| PubMedID: 26174159 |
| Title : Pyridostigmine prevents haemodynamic alterations but does not affect their nycthemeral oscillations in infarcted mice - Correa_2015_Auton.Neurosci_187_50 |
| Author(s) : Correa WG , Durand MT , Becari C , Tezini GC , do Carmo JM , de Oliveira M , Prado CM , Fazan R, Jr. , Salgado HC |
| Ref : Auton Neurosci , 187 :50 , 2015 |
| Abstract : Correa_2015_Auton.Neurosci_187_50 |
| ESTHER : Correa_2015_Auton.Neurosci_187_50 |
| PubMedSearch : Correa_2015_Auton.Neurosci_187_50 |
| PubMedID: 25434306 |
| Title : Paraoxon and Pyridostigmine Interfere with Neural Stem Cell Differentiation - Berrios_2015_Neurochem.Res_40_2091 |
| Author(s) : Berrios VO , Boukli NM , Rodriguez JW , Negraes PD , Schwindt TT , Trujillo CA , Oliveira SL , Cubano LA , Ferchmin PA , Eterovic VA , Ulrich H , Martins AH |
| Ref : Neurochem Res , 40 :2091 , 2015 |
| Abstract : Berrios_2015_Neurochem.Res_40_2091 |
| ESTHER : Berrios_2015_Neurochem.Res_40_2091 |
| PubMedSearch : Berrios_2015_Neurochem.Res_40_2091 |
| PubMedID: 25758980 |
| Title : Effects of cholinergic stimulation with pyridostigmine bromide on chronic chagasic cardiomyopathic mice - de Cuba_2014_Mediators.Inflamm_2014_475946 |
| Author(s) : de Cuba MB , Ribeiro Machado MP , Farnesi TS , Alves AC , Martins LA , de Oliveira LF , Capitelli CS , Leite CF , Vinicius Silva M , Machado JR , Kappel HB , Sales de Campos H , Paiva L , da Silva Gomes NL , Guimaraes Faleiros AC , Britto CF , Savino W , Moreira OC , Rodrigues V, Jr. , Montano N , Lages-Silva E , Ramirez LE , Dias da Silva VJ |
| Ref : Mediators Inflamm , 2014 :475946 , 2014 |
| Abstract : de Cuba_2014_Mediators.Inflamm_2014_475946 |
| ESTHER : de Cuba_2014_Mediators.Inflamm_2014_475946 |
| PubMedSearch : de Cuba_2014_Mediators.Inflamm_2014_475946 |
| PubMedID: 25221388 |
| Title : Pyridostigmine restores cardiac autonomic balance after small myocardial infarction in mice - Durand_2014_PLoS.One_9_e104476 |
| Author(s) : Durand MT , Becari C , Oliveira M , do Carmo JM , Aguiar Silva CA , Prado CM , Fazan R, Jr. , Salgado HC |
| Ref : PLoS ONE , 9 :e104476 , 2014 |
| Abstract : Durand_2014_PLoS.One_9_e104476 |
| ESTHER : Durand_2014_PLoS.One_9_e104476 |
| PubMedSearch : Durand_2014_PLoS.One_9_e104476 |
| PubMedID: 25133392 |
| Title : Pyridostigmine prevents peripheral vascular endothelial dysfunction in rats with myocardial infarction - Qin_2014_Clin.Exp.Pharmacol.Physiol_41_202 |
| Author(s) : Qin F , Lu Y , He X , Zhao M , Bi X , Yu X , Liu J , Zang W |
| Ref : Clinical & Experimental Pharmacology & Physiology , 41 :202 , 2014 |
| Abstract : Qin_2014_Clin.Exp.Pharmacol.Physiol_41_202 |
| ESTHER : Qin_2014_Clin.Exp.Pharmacol.Physiol_41_202 |
| PubMedSearch : Qin_2014_Clin.Exp.Pharmacol.Physiol_41_202 |
| PubMedID: 24471445 |
| Title : The Evaluation of Prophylactic Efficacy of Newly Developed Reversible Inhibitors of Acetylcholinesterase in Soman-Poisoned Mice - A Comparison with Commonly Used Pyridostigmine - Kassa_2014_Basic.Clin.Pharmacol.Toxicol_115_571 |
| Author(s) : Kassa J , Korabecny J , Sepsova V , Tumova M |
| Ref : Basic Clin Pharmacol Toxicol , 115 :571 , 2014 |
| Abstract : Kassa_2014_Basic.Clin.Pharmacol.Toxicol_115_571 |
| ESTHER : Kassa_2014_Basic.Clin.Pharmacol.Toxicol_115_571 |
| PubMedSearch : Kassa_2014_Basic.Clin.Pharmacol.Toxicol_115_571 |
| PubMedID: 24842281 |
| Title : The treatment with pyridostigmine improves the cardiocirculatory function in rats with chronic heart failure - Sabino_2013_Auton.Neurosci_173_58 |
| Author(s) : Sabino JP , da Silva CA , de Melo RF , Fazan R, Jr. , Salgado HC |
| Ref : Auton Neurosci , 173 :58 , 2013 |
| Abstract : Sabino_2013_Auton.Neurosci_173_58 |
| ESTHER : Sabino_2013_Auton.Neurosci_173_58 |
| PubMedSearch : Sabino_2013_Auton.Neurosci_173_58 |
| PubMedID: 23218833 |
| Title : Nanosized sustained-release pyridostigmine bromide microcapsules: process optimization and evaluation of characteristics - Tan_2013_Int.J.Nanomedicine_8_737 |
| Author(s) : Tan Q , Jiang R , Xu M , Liu G , Li S , Zhang J |
| Ref : Int J Nanomedicine , 8 :737 , 2013 |
| Abstract : Tan_2013_Int.J.Nanomedicine_8_737 |
| ESTHER : Tan_2013_Int.J.Nanomedicine_8_737 |
| PubMedSearch : Tan_2013_Int.J.Nanomedicine_8_737 |
| PubMedID: 23459707 |
| Title : Pyridostigmine but not 3,4-diaminopyridine exacerbates ACh receptor loss and myasthenia induced in mice by muscle-specific kinase autoantibody - Morsch_2013_J.Physiol_591_2747 |
| Author(s) : Morsch M , Reddel SW , Ghazanfari N , Toyka KV , Phillips WD |
| Ref : The Journal of Physiology , 591 :2747 , 2013 |
| Abstract : Morsch_2013_J.Physiol_591_2747 |
| ESTHER : Morsch_2013_J.Physiol_591_2747 |
| PubMedSearch : Morsch_2013_J.Physiol_591_2747 |
| PubMedID: 23440963 |
| Title : Increase in parasympathetic tone by pyridostigmine prevents ventricular dysfunction during the onset of heart failure - Lataro_2013_Am.J.Physiol.Regul.Integr.Comp.Physiol_305_R908 |
| Author(s) : Lataro RM , Silva CA , Fazan R, Jr. , Rossi MA , Prado CM , Godinho RO , Salgado HC |
| Ref : American Journal of Physiology Regul Integr Comp Physiol , 305 :R908 , 2013 |
| Abstract : Lataro_2013_Am.J.Physiol.Regul.Integr.Comp.Physiol_305_R908 |
| ESTHER : Lataro_2013_Am.J.Physiol.Regul.Integr.Comp.Physiol_305_R908 |
| PubMedSearch : Lataro_2013_Am.J.Physiol.Regul.Integr.Comp.Physiol_305_R908 |
| PubMedID: 23948774 |
| Title : Persistent Na and K channel dysfunctions after chronic exposure to insecticides and pyridostigmine bromide - Nutter_2013_Neurotoxicol_39C_72 |
| Author(s) : Nutter TJ , Jiang N , Cooper BY |
| Ref : Neurotoxicology , 39C :72 , 2013 |
| Abstract : Nutter_2013_Neurotoxicol_39C_72 |
| ESTHER : Nutter_2013_Neurotoxicol_39C_72 |
| PubMedSearch : Nutter_2013_Neurotoxicol_39C_72 |
| PubMedID: 23994030 |
| Title : Chronic elevation of phosphocholine containing lipids in mice exposed to Gulf War agents pyridostigmine bromide and permethrin - Abdullah_2013_Neurotoxicol.Teratol_40C_74 |
| Author(s) : Abdullah L , Evans JE , Montague H , Reed JM , Moser A , Crynen G , Gonzalez A , Zakirova Z , Ross I , Mullan C , Mullan M , Ait-Ghezala G , Crawford F |
| Ref : Neurotoxicology & Teratology , 40C :74 , 2013 |
| Abstract : Abdullah_2013_Neurotoxicol.Teratol_40C_74 |
| ESTHER : Abdullah_2013_Neurotoxicol.Teratol_40C_74 |
| PubMedSearch : Abdullah_2013_Neurotoxicol.Teratol_40C_74 |
| PubMedID: 24140745 |
| Title : Pyridostigmine bromide protection against acetylcholinesterase inhibition by pesticides - Henderson_2012_J.Biochem.Mol.Toxicol_26_31 |
| Author(s) : Henderson JD , Glucksman G , Leong B , Tigyi A , Ankirskaia A , Siddique I , Lam H , DePeters E , Wilson BW |
| Ref : J Biochem Mol Toxicol , 26 :31 , 2012 |
| Abstract : Henderson_2012_J.Biochem.Mol.Toxicol_26_31 |
| ESTHER : Henderson_2012_J.Biochem.Mol.Toxicol_26_31 |
| PubMedSearch : Henderson_2012_J.Biochem.Mol.Toxicol_26_31 |
| PubMedID: 21972196 |
| Title : A comparison of the efficacy of newly developed reversible inhibitors of acetylcholinesterase with commonly used pyridostigmine as pharmacological pre-treatment of soman-poisoned mice - Kassa_2012_Basic.Clin.Pharmacol.Toxicol_110_322 |
| Author(s) : Kassa J , Musilek K , Koomlova M , Bajgar J |
| Ref : Basic Clin Pharmacol Toxicol , 110 :322 , 2012 |
| Abstract : Kassa_2012_Basic.Clin.Pharmacol.Toxicol_110_322 |
| ESTHER : Kassa_2012_Basic.Clin.Pharmacol.Toxicol_110_322 |
| PubMedSearch : Kassa_2012_Basic.Clin.Pharmacol.Toxicol_110_322 |
| PubMedID: 21981462 |
| Title : Prolonged release matrix tablet of pyridostigmine bromide: formulation and optimization using statistical methods - Bolourchian_2012_Pak.J.Pharm.Sci_25_607 |
| Author(s) : Bolourchian N , Rangchian M , Foroutan SM |
| Ref : Pak J Pharm Sci , 25 :607 , 2012 |
| Abstract : Bolourchian_2012_Pak.J.Pharm.Sci_25_607 |
| ESTHER : Bolourchian_2012_Pak.J.Pharm.Sci_25_607 |
| PubMedSearch : Bolourchian_2012_Pak.J.Pharm.Sci_25_607 |
| PubMedID: 22713949 |
| Title : In vitro kinetic interactions of pyridostigmine, physostigmine and soman with erythrocyte and muscle acetylcholinesterase from different species - Herkert_2011_Toxicol.Lett_206_41 |
| Author(s) : Herkert NM , Thiermann H , Worek F |
| Ref : Toxicol Lett , 206 :41 , 2011 |
| Abstract : Herkert_2011_Toxicol.Lett_206_41 |
| ESTHER : Herkert_2011_Toxicol.Lett_206_41 |
| PubMedSearch : Herkert_2011_Toxicol.Lett_206_41 |
| PubMedID: 21414391 |
| Title : Protection of human muscle acetylcholinesterase from soman by pyridostigmine bromide - Maselli_2011_Muscle.Nerve_43_591 |
| Author(s) : Maselli RA , Henderson JD , Ng J , Follette D , Graves G , Wilson BW |
| Ref : Muscle & Nerve , 43 :591 , 2011 |
| Abstract : Maselli_2011_Muscle.Nerve_43_591 |
| ESTHER : Maselli_2011_Muscle.Nerve_43_591 |
| PubMedSearch : Maselli_2011_Muscle.Nerve_43_591 |
| PubMedID: 21404290 |
| Title : The effect of pyridostigmine on bispectral index during recovery from sevoflurane anesthesia - Jeong_2011_Korean.J.Anesthesiol_61_460 |
| Author(s) : Jeong SJ , Han JI , Baik HJ , Lee H , Lee GY , Kim JH |
| Ref : Korean J Anesthesiol , 61 :460 , 2011 |
| Abstract : Jeong_2011_Korean.J.Anesthesiol_61_460 |
| ESTHER : Jeong_2011_Korean.J.Anesthesiol_61_460 |
| PubMedSearch : Jeong_2011_Korean.J.Anesthesiol_61_460 |
| PubMedID: 22220221 |
| Title : Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience - Kanjwal_2011_Pacing.Clin.Electrophysiol_34_750 |
| Author(s) : Kanjwal K , Karabin B , Sheikh M , Elmer L , Kanjwal Y , Saeed B , Grubb BP |
| Ref : Pacing Clin Electrophysiol , 34 :750 , 2011 |
| Abstract : Kanjwal_2011_Pacing.Clin.Electrophysiol_34_750 |
| ESTHER : Kanjwal_2011_Pacing.Clin.Electrophysiol_34_750 |
| PubMedSearch : Kanjwal_2011_Pacing.Clin.Electrophysiol_34_750 |
| PubMedID: 21410722 |
| Title : Treatment of myasthenia gravis: focus on pyridostigmine - Maggi_2011_Clin.Drug.Investig_31_691 |
| Author(s) : Maggi L , Mantegazza R |
| Ref : Clin Drug Investig , 31 :691 , 2011 |
| Abstract : Maggi_2011_Clin.Drug.Investig_31_691 |
| ESTHER : Maggi_2011_Clin.Drug.Investig_31_691 |
| PubMedSearch : Maggi_2011_Clin.Drug.Investig_31_691 |
| PubMedID: 21815707 |
| Title : In vitro kinetic interactions of DEET, pyridostigmine and organophosphorus pesticides with human cholinesterases - Wille_2011_Chem.Biol.Interact_190_79 |
| Author(s) : Wille T , Thiermann H , Worek F |
| Ref : Chemico-Biological Interactions , 190 :79 , 2011 |
| Abstract : Wille_2011_Chem.Biol.Interact_190_79 |
| ESTHER : Wille_2011_Chem.Biol.Interact_190_79 |
| PubMedSearch : Wille_2011_Chem.Biol.Interact_190_79 |
| PubMedID: 21354413 |
| Title : In vitro kinetic interactions of DEET, pyridostigmine and organophosphorus pesticides with human cholinesterases - Response to the letter to the editor - |
| Author(s) : Wille T , Thiermann H , Worek F |
| Ref : Chemico-Biological Interactions , 193 :108 , 2011 |
| PubMedID: |
| Title : Continuous administration of pyridostigmine improves immobilization-induced neuromuscular weakness - Frick_2010_Crit.Care.Med_38_922 |
| Author(s) : Frick CG , Helming M , Martyn JA , Blobner M , Fink H |
| Ref : Critical Care Medicine , 38 :922 , 2010 |
| Abstract : Frick_2010_Crit.Care.Med_38_922 |
| ESTHER : Frick_2010_Crit.Care.Med_38_922 |
| PubMedSearch : Frick_2010_Crit.Care.Med_38_922 |
| PubMedID: 20009758 |
| Title : Protection by pyridostigmine bromide of marmoset hemi-diaphragm acetylcholinesterase activity after soman exposure - Haigh_2010_Chem.Biol.Interact_187_416 |
| Author(s) : Haigh JR , Adler M , Apland JP , Deshpande SS , Barham CB , Desmond P , Koplovitz I , Lenz DE , Gordon RK |
| Ref : Chemico-Biological Interactions , 187 :416 , 2010 |
| Abstract : Haigh_2010_Chem.Biol.Interact_187_416 |
| ESTHER : Haigh_2010_Chem.Biol.Interact_187_416 |
| PubMedSearch : Haigh_2010_Chem.Biol.Interact_187_416 |
| PubMedID: 20144889 |
| Title : Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure - Shibao_2010_Hypertension_56_847 |
| Author(s) : Shibao C , Okamoto LE , Gamboa A , Yu C , Diedrich A , Raj SR , Robertson D , Biaggioni I |
| Ref : Hypertension , 56 :847 , 2010 |
| Abstract : Shibao_2010_Hypertension_56_847 |
| ESTHER : Shibao_2010_Hypertension_56_847 |
| PubMedSearch : Shibao_2010_Hypertension_56_847 |
| PubMedID: 20837887 |
| Title : Benefits from sustained-release pyridostigmine bromide in myasthenia gravis: results of a prospective multicenter open-label trial - Sieb_2010_Clin.Neurol.Neurosurg_112_781 |
| Author(s) : Sieb JP , Kohler W |
| Ref : Clin Neurol Neurosurg , 112 :781 , 2010 |
| Abstract : Sieb_2010_Clin.Neurol.Neurosurg_112_781 |
| ESTHER : Sieb_2010_Clin.Neurol.Neurosurg_112_781 |
| PubMedSearch : Sieb_2010_Clin.Neurol.Neurosurg_112_781 |
| PubMedID: 20663605 |
| Title : The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine - O'Dea_2010_Colorectal.Dis_12_540 |
| Author(s) : O'Dea CJ , Brookes JH , Wattchow DA |
| Ref : Colorectal Dis , 12 :540 , 2010 |
| Abstract : O'Dea_2010_Colorectal.Dis_12_540 |
| ESTHER : O'Dea_2010_Colorectal.Dis_12_540 |
| PubMedSearch : O'Dea_2010_Colorectal.Dis_12_540 |
| PubMedID: 19508545 |
| Title : Relief from episodic weakness with pyridostigmine in paramyotonia congenita: a family study - Khadilkar_2010_Muscle.Nerve_41_133 |
| Author(s) : Khadilkar SV , Singh RK , Mansukhani KA , Urtizberea JA , Sternberg D |
| Ref : Muscle & Nerve , 41 :133 , 2010 |
| Abstract : Khadilkar_2010_Muscle.Nerve_41_133 |
| ESTHER : Khadilkar_2010_Muscle.Nerve_41_133 |
| PubMedSearch : Khadilkar_2010_Muscle.Nerve_41_133 |
| PubMedID: 19768756 |
| Title : Acute electrophysiologic consequences of pyridostigmine inhibition of cholinesterase in humans - Zimerman_2010_Braz.J.Med.Biol.Res_43_211 |
| Author(s) : Zimerman LI , Liberman A , Castro RR , Ribeiro JP , Nobrega AC |
| Ref : Brazilian Journal of Medical & Biological Research , 43 :211 , 2010 |
| Abstract : Zimerman_2010_Braz.J.Med.Biol.Res_43_211 |
| ESTHER : Zimerman_2010_Braz.J.Med.Biol.Res_43_211 |
| PubMedSearch : Zimerman_2010_Braz.J.Med.Biol.Res_43_211 |
| PubMedID: 20084332 |
| Title : Gulf War illness: Effects of repeated stress and pyridostigmine treatment on blood-brain barrier permeability and cholinesterase activity in rat brain - Amourette_2009_Behav.Brain.Res_203_207 |
| Author(s) : Amourette C , Lamproglou I , Barbier L , Fauquette W , Zoppe A , Viret R , Diserbo M |
| Ref : Behavioural Brain Research , 203 :207 , 2009 |
| Abstract : Amourette_2009_Behav.Brain.Res_203_207 |
| ESTHER : Amourette_2009_Behav.Brain.Res_203_207 |
| PubMedSearch : Amourette_2009_Behav.Brain.Res_203_207 |
| PubMedID: 19433115 |
| Title : The effect of oral buspirone, pyridostigmine, and bethanechol on esophageal function evaluated with combined multichannel esophageal impedance-manometry in healthy volunteers - Blonski_2009_J.Clin.Gastroenterol_43_253 |
| Author(s) : Blonski W , Vela MF , Freeman J , Sharma N , Castell DO |
| Ref : J Clin Gastroenterol , 43 :253 , 2009 |
| Abstract : Blonski_2009_J.Clin.Gastroenterol_43_253 |
| ESTHER : Blonski_2009_J.Clin.Gastroenterol_43_253 |
| PubMedSearch : Blonski_2009_J.Clin.Gastroenterol_43_253 |
| PubMedID: 18987553 |
| Title : Repeated stress in combination with pyridostigmine Part II: changes in cerebral gene expression - Barbier_2009_Behav.Brain.Res_197_292 |
| Author(s) : Barbier L , Diserbo M , Lamproglou I , Amourette C , Peinnequin A , Fauquette W |
| Ref : Behavioural Brain Research , 197 :292 , 2009 |
| Abstract : Barbier_2009_Behav.Brain.Res_197_292 |
| ESTHER : Barbier_2009_Behav.Brain.Res_197_292 |
| PubMedSearch : Barbier_2009_Behav.Brain.Res_197_292 |
| PubMedID: 18796314 |
| Title : Retrospective population pharmacokinetic\/pharmacodynamic analysis of pyridostigmine, a cholinesterase inhibitor, in Chinese males - Seng_2009_J.Pharm.Pharmacol_61_1187 |
| Author(s) : Seng KY , Loke WK , Moochhala S , Zhao B , Lee JD |
| Ref : J Pharm Pharmacol , 61 :1187 , 2009 |
| Abstract : Seng_2009_J.Pharm.Pharmacol_61_1187 |
| ESTHER : Seng_2009_J.Pharm.Pharmacol_61_1187 |
| PubMedSearch : Seng_2009_J.Pharm.Pharmacol_61_1187 |
| PubMedID: 19703368 |
| Title : Acetylcholine-esterase inhibitor pyridostigmine decreases T cell overactivation in patients infected by HIV - Valdes-Ferrer_2009_AIDS.Res.Hum.Retroviruses_25_749 |
| Author(s) : Valdes-Ferrer SI , Crispin JC , Belaunzaran PF , Cantu-Brito CG , Sierra-Madero J , Alcocer-Varela J |
| Ref : AIDS Res Hum Retroviruses , 25 :749 , 2009 |
| Abstract : Valdes-Ferrer_2009_AIDS.Res.Hum.Retroviruses_25_749 |
| ESTHER : Valdes-Ferrer_2009_AIDS.Res.Hum.Retroviruses_25_749 |
| PubMedSearch : Valdes-Ferrer_2009_AIDS.Res.Hum.Retroviruses_25_749 |
| PubMedID: 19645607 |
| Title : Effect of pyridostigmine (Mestinon) on human platelet aggregation - Leong_2009_Malays.J.Pathol_31_45 |
| Author(s) : Leong CF , Aini-Ardena M , Cheong SK , Norris N |
| Ref : Malays Journal of Pathology , 31 :45 , 2009 |
| Abstract : Leong_2009_Malays.J.Pathol_31_45 |
| ESTHER : Leong_2009_Malays.J.Pathol_31_45 |
| PubMedSearch : Leong_2009_Malays.J.Pathol_31_45 |
| PubMedID: 19694313 |
| Title : Pilot study of pyridostigmine in constipated patients with autonomic neuropathy - Bharucha_2008_Clin.Auton.Res_18_194 |
| Author(s) : Bharucha AE , Low PA , Camilleri M , Burton D , Gehrking TL , Zinsmeister AR |
| Ref : Clin Auton Res , 18 :194 , 2008 |
| Abstract : Bharucha_2008_Clin.Auton.Res_18_194 |
| ESTHER : Bharucha_2008_Clin.Auton.Res_18_194 |
| PubMedSearch : Bharucha_2008_Clin.Auton.Res_18_194 |
| PubMedID: 18622640 |
| Title : Cholinergic stimulation with pyridostigmine prevents the impairment in ventricular function during mental stress in coronary artery disease patients - Nobrega_2008_Int.J.Cardiol_125_418 |
| Author(s) : Nobrega AC , Loures DL , Pontes PV , Sant'anna ID , Mesquita ET |
| Ref : Int J Cardiol , 125 :418 , 2008 |
| Abstract : Nobrega_2008_Int.J.Cardiol_125_418 |
| ESTHER : Nobrega_2008_Int.J.Cardiol_125_418 |
| PubMedSearch : Nobrega_2008_Int.J.Cardiol_125_418 |
| PubMedID: 17397949 |
| Title : Effects of combined, multiple stressors on pyridostigmine-induced acute toxicity in rats - Baireddy_2007_Arch.Toxicol_81_283 |
| Author(s) : Baireddy P , Mirajkar N , Nallapaneni A , Singleton N , Pope CN |
| Ref : Archives of Toxicology , 81 :283 , 2007 |
| Abstract : Baireddy_2007_Arch.Toxicol_81_283 |
| ESTHER : Baireddy_2007_Arch.Toxicol_81_283 |
| PubMedSearch : Baireddy_2007_Arch.Toxicol_81_283 |
| PubMedID: 16944100 |
| Title : Comparison of two pre-exposure treatment regimens in acute organophosphate (paraoxon) poisoning in rats: tiapride vs. pyridostigmine - Petroianu_2007_Toxicol.Appl.Pharmacol_219_235 |
| Author(s) : Petroianu GA , Hasan MY , Nurulain SM , Arafat K , Sheen R , Nagelkerke N |
| Ref : Toxicol Appl Pharmacol , 219 :235 , 2007 |
| Abstract : Petroianu_2007_Toxicol.Appl.Pharmacol_219_235 |
| ESTHER : Petroianu_2007_Toxicol.Appl.Pharmacol_219_235 |
| PubMedSearch : Petroianu_2007_Toxicol.Appl.Pharmacol_219_235 |
| PubMedID: 17056080 |
| Title : Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes - Dewland_2007_Am.J.Physiol.Heart.Circ.Physiol_293_H86 |
| Author(s) : Dewland TA , Androne AS , Lee FA , Lampert RJ , Katz SD |
| Ref : American Journal of Physiology Heart Circ Physiol , 293 :H86 , 2007 |
| Abstract : Dewland_2007_Am.J.Physiol.Heart.Circ.Physiol_293_H86 |
| ESTHER : Dewland_2007_Am.J.Physiol.Heart.Circ.Physiol_293_H86 |
| PubMedSearch : Dewland_2007_Am.J.Physiol.Heart.Circ.Physiol_293_H86 |
| PubMedID: 17322413 |
| Title : Pyridostigmine in the treatment of orthostatic intolerance - Gales_2007_Ann.Pharmacother_41_314 |
| Author(s) : Gales BJ , Gales MA |
| Ref : Annals of Pharmacotherapy , 41 :314 , 2007 |
| Abstract : Gales_2007_Ann.Pharmacother_41_314 |
| ESTHER : Gales_2007_Ann.Pharmacother_41_314 |
| PubMedSearch : Gales_2007_Ann.Pharmacother_41_314 |
| PubMedID: 17284509 |
| Title : An investigation of acetylcholine released in skeletal muscle and protein unbound drug released in blood based on the pyridostigmine bromide (pretreatment drug) sustained-release pellets by microdialysis technique in the rabbit model - Huang_2007_Neurosci.Lett_416_302 |
| Author(s) : Huang YT , Cheng CJ , Lai TF , Tsai TR , Tsai TH , Chuo WH , Cham TM |
| Ref : Neuroscience Letters , 416 :302 , 2007 |
| Abstract : Huang_2007_Neurosci.Lett_416_302 |
| ESTHER : Huang_2007_Neurosci.Lett_416_302 |
| PubMedSearch : Huang_2007_Neurosci.Lett_416_302 |
| PubMedID: 17336457 |
| Title : [Pyridostigmine in the treatment of primary orthostatic hypotension] - Monge_2007_Neurologia_22_260 |
| Author(s) : Monge Argiles JA , Leiva Santana C |
| Ref : Neurologia , 22 :260 , 2007 |
| Abstract : Monge_2007_Neurologia_22_260 |
| ESTHER : Monge_2007_Neurologia_22_260 |
| PubMedSearch : Monge_2007_Neurologia_22_260 |
| PubMedID: 17492522 |
| Title : Autoimmune gastrointestinal dysmotility treated successfully with pyridostigmine - Pasha_2006_Gastroenterology_131_1592 |
| Author(s) : Pasha SF , Lunsford TN , Lennon VA |
| Ref : Gastroenterology , 131 :1592 , 2006 |
| Abstract : Pasha_2006_Gastroenterology_131_1592 |
| ESTHER : Pasha_2006_Gastroenterology_131_1592 |
| PubMedSearch : Pasha_2006_Gastroenterology_131_1592 |
| PubMedID: 17101331 |
| Title : Pyridostigmine reduces QTc interval during recovery from maximal exercise in ischemic heart disease - Castro_2006_Int.J.Cardiol_107_138 |
| Author(s) : Castro RR , Serra SM , Porphirio G , Mendes FS , Oliveira LP , Nobrega AC |
| Ref : Int J Cardiol , 107 :138 , 2006 |
| Abstract : Castro_2006_Int.J.Cardiol_107_138 |
| ESTHER : Castro_2006_Int.J.Cardiol_107_138 |
| PubMedSearch : Castro_2006_Int.J.Cardiol_107_138 |
| PubMedID: 16337518 |
| Title : Effect of pyridostigmine, pralidoxime and their combination on survival and cholinesterase activity in rats exposed to the organophosphate paraoxon - Petroianu_2006_Arch.Toxicol_80_777 |
| Author(s) : Petroianu GA , Nurulain SM , Arafat K , Rajan S , Hasan MY |
| Ref : Archives of Toxicology , 80 :777 , 2006 |
| Abstract : Petroianu_2006_Arch.Toxicol_80_777 |
| ESTHER : Petroianu_2006_Arch.Toxicol_80_777 |
| PubMedSearch : Petroianu_2006_Arch.Toxicol_80_777 |
| PubMedID: 16598495 |
| Title : Ranitidine in acute high-dose organophosphate exposure in rats: effect of the time-point of administration and comparison with pyridostigmine - Petroianu_2006_Basic.Clin.Pharmacol.Toxicol_99_312 |
| Author(s) : Petroianu GA , Hasan MY , Nurulain SM , Shafiullah M , Sheen R , Nagelkerke N |
| Ref : Basic Clin Pharmacol Toxicol , 99 :312 , 2006 |
| Abstract : Petroianu_2006_Basic.Clin.Pharmacol.Toxicol_99_312 |
| ESTHER : Petroianu_2006_Basic.Clin.Pharmacol.Toxicol_99_312 |
| PubMedSearch : Petroianu_2006_Basic.Clin.Pharmacol.Toxicol_99_312 |
| PubMedID: 17040217 |
| Title : Development of methods to measure humoral immune responses against selected antigens in the common marmoset (Callithrix jacchus) and the effect of pyridostigmine bromide administration - Griffiths_2006_Int.Immunopharmacol_6_1755 |
| Author(s) : Griffiths GD , Hornby RJ , Jagger CP , Brown AP , Stoten A , Pearce PC , Scott L , Pritchard DI |
| Ref : Int Immunopharmacol , 6 :1755 , 2006 |
| Abstract : Griffiths_2006_Int.Immunopharmacol_6_1755 |
| ESTHER : Griffiths_2006_Int.Immunopharmacol_6_1755 |
| PubMedSearch : Griffiths_2006_Int.Immunopharmacol_6_1755 |
| PubMedID: 17052666 |
| Title : Development of miotic cross-tolerance between pyridostigmine and sarin vapor - Dabisch_2006_J.Ocul.Pharmacol.Ther_22_323 |
| Author(s) : Dabisch PA , Davis EA , Horsmon MS , Mioduszewski RJ |
| Ref : J Ocul Pharmacol Ther , 22 :323 , 2006 |
| Abstract : Dabisch_2006_J.Ocul.Pharmacol.Ther_22_323 |
| ESTHER : Dabisch_2006_J.Ocul.Pharmacol.Ther_22_323 |
| PubMedSearch : Dabisch_2006_J.Ocul.Pharmacol.Ther_22_323 |
| PubMedID: 17076626 |
| Title : Pyridostigmine in autonomic failure: can we treat postural hypotension and bladder dysfunction with one drug? - Yamamoto_2006_Clin.Auton.Res_16_296 |
| Author(s) : Yamamoto T , Sakakibara R , Yamanaka Y , Uchiyama T , Asahina M , Liu Z , Ito T , Koyama Y , Awa Y , Yamamoto K , Kinou M , Hattori T |
| Ref : Clin Auton Res , 16 :296 , 2006 |
| Abstract : Yamamoto_2006_Clin.Auton.Res_16_296 |
| ESTHER : Yamamoto_2006_Clin.Auton.Res_16_296 |
| PubMedSearch : Yamamoto_2006_Clin.Auton.Res_16_296 |
| PubMedID: 16862395 |
| Title : Determination of pyridostigmine bromide and its metabolites in biological samples - Zhao_2006_J.Pharm.Pharm.Sci_9_71 |
| Author(s) : Zhao B , Moochhala SM , Lu J , Tan D , Lai MH |
| Ref : J Pharm Pharm Sci , 9 :71 , 2006 |
| Abstract : Zhao_2006_J.Pharm.Pharm.Sci_9_71 |
| ESTHER : Zhao_2006_J.Pharm.Pharm.Sci_9_71 |
| PubMedSearch : Zhao_2006_J.Pharm.Pharm.Sci_9_71 |
| PubMedID: 16849010 |
| Title : Inhibition of guinea pig hemi-diaphragm acetylcholinesterase activity by pyridostigmine bromide and protection against soman toxicity - |
| Author(s) : Haigh JR , Johnston SR , Peters BM , Doctor BP , Gordon RK , Adler M , Gall KJ , Deshpande SS |
| Ref : Chemico-Biological Interactions , 157-158 :381 , 2005 |
| PubMedID: 16429510 |
| Title : Oral administration of pyridostigmine bromide and huperzine A protects human whole blood cholinesterases from ex vivo exposure to soman - Gordon_2005_Chem.Biol.Interact_157-158_239 |
| Author(s) : Gordon RK , Haigh JR , Garcia GE , Feaster SR , Riel MA , Lenz DE , Aisen PS , Doctor BP |
| Ref : Chemico-Biological Interactions , 157-158 :239 , 2005 |
| Abstract : Gordon_2005_Chem.Biol.Interact_157-158_239 |
| ESTHER : Gordon_2005_Chem.Biol.Interact_157-158_239 |
| PubMedSearch : Gordon_2005_Chem.Biol.Interact_157-158_239 |
| PubMedID: 16256090 |
| Title : A T-cell-dependent humoral immune response is preserved during the administration of the nerve agent pre-treatment pyridostigmine bromide in a murine model - Griffiths_2005_Int.Immunopharmacol_5_525 |
| Author(s) : Griffiths GD , Telford G , Hooi DS , Cook DL , Wilkinson LJ , Green CA , Pritchard DI |
| Ref : Int Immunopharmacol , 5 :525 , 2005 |
| Abstract : Griffiths_2005_Int.Immunopharmacol_5_525 |
| ESTHER : Griffiths_2005_Int.Immunopharmacol_5_525 |
| PubMedSearch : Griffiths_2005_Int.Immunopharmacol_5_525 |
| PubMedID: 15683849 |
| Title : Cholinergic stimulation with pyridostigmine protects against exercise induced myocardial ischaemia - Castro_2004_Heart_90_1119 |
| Author(s) : Castro RR , Porphirio G , Serra SM , Nobrega AC |
| Ref : Heart , 90 :1119 , 2004 |
| Abstract : Castro_2004_Heart_90_1119 |
| ESTHER : Castro_2004_Heart_90_1119 |
| PubMedSearch : Castro_2004_Heart_90_1119 |
| PubMedID: 15367503 |
| Title : Pyridostigmine bromide (PYR) alters immune function in B6C3F1 mice - Peden-Adams_2004_Immunopharmacol.Immunotoxicol_26_1 |
| Author(s) : Peden-Adams MM , Dudley AC , EuDaly JG , Allen CT , Gilkeson GS , Keil DE |
| Ref : Immunopharmacol Immunotoxicol , 26 :1 , 2004 |
| Abstract : Peden-Adams_2004_Immunopharmacol.Immunotoxicol_26_1 |
| ESTHER : Peden-Adams_2004_Immunopharmacol.Immunotoxicol_26_1 |
| PubMedSearch : Peden-Adams_2004_Immunopharmacol.Immunotoxicol_26_1 |
| PubMedID: 15106728 |
| Title : Co-exposure to pyridostigmine bromide, DEET, and\/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity - Abou-Donia_2004_Pharmacol.Biochem.Behav_77_253 |
| Author(s) : Abou-Donia MB , Dechkovskaia AM , Goldstein LB , Abdel-Rahman A , Bullman SL , Khan WA |
| Ref : Pharmacol Biochem Behav , 77 :253 , 2004 |
| Abstract : Abou-Donia_2004_Pharmacol.Biochem.Behav_77_253 |
| ESTHER : Abou-Donia_2004_Pharmacol.Biochem.Behav_77_253 |
| PubMedSearch : Abou-Donia_2004_Pharmacol.Biochem.Behav_77_253 |
| PubMedID: 14751452 |
| Title : Cholinergic stimulation with pyridostigmine increases heart rate variability and baroreflex sensitivity in rats - Soares_2004_Auton.Neurosci_113_24 |
| Author(s) : Soares PP , da Nobrega AC , Ushizima MR , Irigoyen MC |
| Ref : Auton Neurosci , 113 :24 , 2004 |
| Abstract : Soares_2004_Auton.Neurosci_113_24 |
| ESTHER : Soares_2004_Auton.Neurosci_113_24 |
| PubMedSearch : Soares_2004_Auton.Neurosci_113_24 |
| PubMedID: 15296792 |
| Title : Interaction between stress and pyridostigmine pretreatment in the mouse. - |
| Author(s) : Taysse L , Delamanche IS , Perrier NA , Breton P |
| Ref : Cholinergic Mechanisms, CRC Press :729 , 2004 |
| PubMedID: |
| Title : Persistent\/delayed toxic effects of low-dose sarin and pyridostigmine under physical stress (exercise) in mice - Husain_2004_Indian.J.Physiol.Pharmacol_48_150 |
| Author(s) : Husain K , Somani SM |
| Ref : Indian Journal de Physiologie Pharmacol , 48 :150 , 2004 |
| Abstract : Husain_2004_Indian.J.Physiol.Pharmacol_48_150 |
| ESTHER : Husain_2004_Indian.J.Physiol.Pharmacol_48_150 |
| PubMedSearch : Husain_2004_Indian.J.Physiol.Pharmacol_48_150 |
| PubMedID: 15521554 |
| Title : An in vitro investigation of the effects of the nerve agent pretreatment pyridostigmine bromide on human peripheral blood T-cell function - Telford_2004_Int.Immunopharmacol_4_1455 |
| Author(s) : Telford G , Wilkinson LJ , Hooi DS , Worrall V , Green AC , Cook DL , Pritchard DI , Griffiths GD |
| Ref : Int Immunopharmacol , 4 :1455 , 2004 |
| Abstract : Telford_2004_Int.Immunopharmacol_4_1455 |
| ESTHER : Telford_2004_Int.Immunopharmacol_4_1455 |
| PubMedSearch : Telford_2004_Int.Immunopharmacol_4_1455 |
| PubMedID: 15351315 |
| Title : Interactive effects of paraoxon and pyridostigmine on blood-brain barrier integrity and cholinergic toxicity - Song_2004_Toxicol.Sci_78_241 |
| Author(s) : Song X , Pope C , Murthy R , Shaikh J , Lal B , Bressler JP |
| Ref : Toxicol Sci , 78 :241 , 2004 |
| Abstract : Song_2004_Toxicol.Sci_78_241 |
| ESTHER : Song_2004_Toxicol.Sci_78_241 |
| PubMedSearch : Song_2004_Toxicol.Sci_78_241 |
| PubMedID: 14976354 |
| Title : Effect of pyridostigmine administration on acetylcholinesterase and choline acetyltransferase activity in guinea pig striatum and cerebellum. - |
| Author(s) : Smith ME , Lintern MC , Brewer CJ , Wetherell JR |
| Ref : Cholinergic Mechanisms, CRC Press :711 , 2004 |
| PubMedID: |
| Title : Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum - Abdel-Rahman_2004_J.Toxicol.Environ.Health.A_67_163 |
| Author(s) : Abdel-Rahman A , Abou-Donia S , El-Masry E , Shetty A , Abou-Donia MB |
| Ref : J Toxicol Environ Health A , 67 :163 , 2004 |
| Abstract : Abdel-Rahman_2004_J.Toxicol.Environ.Health.A_67_163 |
| ESTHER : Abdel-Rahman_2004_J.Toxicol.Environ.Health.A_67_163 |
| PubMedSearch : Abdel-Rahman_2004_J.Toxicol.Environ.Health.A_67_163 |
| PubMedID: 14675905 |
| Title : Facile synthesis and initial PET imaging of novel potential heart acetylcholinesterase imaging agents [11C]pyridostigmine and its analogs - Wang_2004_Nucl.Med.Biol_31_957 |
| Author(s) : Wang JQ , Miller MA , Fei X , Stone KL , Lopshire JC , Groh WJ , Zipes DP , Hutchins GD , Zheng QH |
| Ref : Nucl Med Biol , 31 :957 , 2004 |
| Abstract : Wang_2004_Nucl.Med.Biol_31_957 |
| ESTHER : Wang_2004_Nucl.Med.Biol_31_957 |
| PubMedSearch : Wang_2004_Nucl.Med.Biol_31_957 |
| PubMedID: 15464398 |
| Title : Combined forced running stress and subclinical paraoxon exposure have little effect on pyridostigmine-induced acute toxicity in rats - Shaikh_2003_Toxicology_190_221 |
| Author(s) : Shaikh J , Pope CN |
| Ref : Toxicology , 190 :221 , 2003 |
| Abstract : Shaikh_2003_Toxicology_190_221 |
| ESTHER : Shaikh_2003_Toxicology_190_221 |
| PubMedSearch : Shaikh_2003_Toxicology_190_221 |
| PubMedID: 12927376 |
| Title : Low concentrations of pyridostigmine prevent soman-induced inhibition of GABAergic transmission in the central nervous system: involvement of muscarinic receptors - Santos_2003_J.Pharmacol.Exp.Ther_304_254 |
| Author(s) : Santos MD , Pereira EF , Aracava Y , Castro NG , Fawcett WP , Randall WR , Albuquerque EX |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 304 :254 , 2003 |
| Abstract : Santos_2003_J.Pharmacol.Exp.Ther_304_254 |
| ESTHER : Santos_2003_J.Pharmacol.Exp.Ther_304_254 |
| PubMedSearch : Santos_2003_J.Pharmacol.Exp.Ther_304_254 |
| PubMedID: 12490599 |
| Title : Acetylcholinesterase inhibition with pyridostigmine improves heart rate recovery after maximal exercise in patients with chronic heart failure - Androne_2003_Heart_89_854 |
| Author(s) : Androne AS , Hryniewicz K , Goldsmith R , Arwady A , Katz SD |
| Ref : Heart , 89 :854 , 2003 |
| Abstract : Androne_2003_Heart_89_854 |
| ESTHER : Androne_2003_Heart_89_854 |
| PubMedSearch : Androne_2003_Heart_89_854 |
| PubMedID: 12860856 |
| Title : Efficacy of a half dose of oral pyridostigmine in the treatment of chronic fatigue syndrome: three case reports - Kawamura_2003_Pathophysiology_9_189 |
| Author(s) : Kawamura Y , Kihara M , Nishimoto K , Taki M |
| Ref : Pathophysiology , 9 :189 , 2003 |
| Abstract : Kawamura_2003_Pathophysiology_9_189 |
| ESTHER : Kawamura_2003_Pathophysiology_9_189 |
| PubMedSearch : Kawamura_2003_Pathophysiology_9_189 |
| PubMedID: 14567934 |
| Title : Effects of daily stress or repeated paraoxon exposures on subacute pyridostigmine toxicity in rats - Shaikh_2003_Arch.Toxicol_77_576 |
| Author(s) : Shaikh J , Karanth S , Chakraborty D , Pruett S , Pope CN |
| Ref : Archives of Toxicology , 77 :576 , 2003 |
| Abstract : Shaikh_2003_Arch.Toxicol_77_576 |
| ESTHER : Shaikh_2003_Arch.Toxicol_77_576 |
| PubMedSearch : Shaikh_2003_Arch.Toxicol_77_576 |
| PubMedID: 14574445 |
| Title : Cholinergic stimulation with pyridostigmine reduces ventricular arrhythmia and enhances heart rate variability in heart failure - Behling_2003_Am.Heart.J_146_494 |
| Author(s) : Behling A , Moraes RS , Rohde LE , Ferlin EL , Nobrega AC , Ribeiro JP |
| Ref : American Heart Journal , 146 :494 , 2003 |
| Abstract : Behling_2003_Am.Heart.J_146_494 |
| ESTHER : Behling_2003_Am.Heart.J_146_494 |
| PubMedSearch : Behling_2003_Am.Heart.J_146_494 |
| PubMedID: 12947369 |
| Title : Effect of chronic pyridostigmine bromide treatment on cardiovascular and behavioral parameters in mice - Bernatova_2003_Pharmacol.Biochem.Behav_74_901 |
| Author(s) : Bernatova I , Dubovicky M , Price WA , Grubbs RD , Lucot JB , Morris M |
| Ref : Pharmacol Biochem Behav , 74 :901 , 2003 |
| Abstract : Bernatova_2003_Pharmacol.Biochem.Behav_74_901 |
| ESTHER : Bernatova_2003_Pharmacol.Biochem.Behav_74_901 |
| PubMedSearch : Bernatova_2003_Pharmacol.Biochem.Behav_74_901 |
| PubMedID: 12667905 |
| Title : Pyridophens: binary pyridostigmine-aprophen prodrugs with differential inhibition of acetylcholinesterase, butyrylcholinesterase, and muscarinic receptors - Leader_2002_J.Med.Chem_45_902 |
| Author(s) : Leader H , Wolfe AD , Chiang PK , Gordon RK |
| Ref : Journal of Medicinal Chemistry , 45 :902 , 2002 |
| Abstract : Leader_2002_J.Med.Chem_45_902 |
| ESTHER : Leader_2002_J.Med.Chem_45_902 |
| PubMedSearch : Leader_2002_J.Med.Chem_45_902 |
| PubMedID: 11831902 |
| Title : Sensorimotor deficit and cholinergic changes following coexposure with pyridostigmine bromide and sarin in rats - Abou-Donia_2002_Toxicol.Sci_66_148 |
| Author(s) : Abou-Donia MB , Dechkovskaia AM , Goldstein LB , Bullman SL , Khan WA |
| Ref : Toxicol Sci , 66 :148 , 2002 |
| Abstract : Abou-Donia_2002_Toxicol.Sci_66_148 |
| ESTHER : Abou-Donia_2002_Toxicol.Sci_66_148 |
| PubMedSearch : Abou-Donia_2002_Toxicol.Sci_66_148 |
| PubMedID: 11861982 |
| Title : Subchronic administration of pyridostigmine or huperzine to primates: compared efficacy against soman toxicity - Lallement_2002_Drug.Chem.Toxicol_25_309 |
| Author(s) : Lallement G , Demoncheaux JP , Foquin A , Baubichon D , Galonnier M , Clarencon D , Dorandeu F |
| Ref : Drug & Chemical Toxicology , 25 :309 , 2002 |
| Abstract : Lallement_2002_Drug.Chem.Toxicol_25_309 |
| ESTHER : Lallement_2002_Drug.Chem.Toxicol_25_309 |
| PubMedSearch : Lallement_2002_Drug.Chem.Toxicol_25_309 |
| PubMedID: 12173251 |
| Title : Cardiorespiratory Effects Following Acute Exposure to Pyridostigmine Bromide and\/or N,N-Diethyl-m-toluamide (DEET) in Rats - Chaney_2002_Int.J.Toxicol_21_287 |
| Author(s) : Chaney LA , Rockhold RW , Hume AS |
| Ref : Int J Toxicol , 21 :287 , 2002 |
| Abstract : Chaney_2002_Int.J.Toxicol_21_287 |
| ESTHER : Chaney_2002_Int.J.Toxicol_21_287 |
| PubMedSearch : Chaney_2002_Int.J.Toxicol_21_287 |
| PubMedID: 12171630 |
| Title : Altered hypothalamic cholinergic responses in patients with nonulcer dyspepsia: a study of pyridostigmine-stimulated growth hormone release - Dinan_2002_Am.J.Gastroenterol_97_1937 |
| Author(s) : Dinan TG , Scott LV , Brady D , McNamara D , Keeling PW |
| Ref : Am J Gastroenterol , 97 :1937 , 2002 |
| Abstract : Dinan_2002_Am.J.Gastroenterol_97_1937 |
| ESTHER : Dinan_2002_Am.J.Gastroenterol_97_1937 |
| PubMedSearch : Dinan_2002_Am.J.Gastroenterol_97_1937 |
| PubMedID: 12190157 |
| Title : Physiological and performance effects of pyridostigmine bromide in healthy volunteers: a dose-response study - Cook_2002_Psychopharmacology.(Berl)_162_186 |
| Author(s) : Cook MR , Graham C , Sastre A , Gerkovich MM |
| Ref : Psychopharmacology (Berl) , 162 :186 , 2002 |
| Abstract : Cook_2002_Psychopharmacology.(Berl)_162_186 |
| ESTHER : Cook_2002_Psychopharmacology.(Berl)_162_186 |
| PubMedSearch : Cook_2002_Psychopharmacology.(Berl)_162_186 |
| PubMedID: 12110996 |
| Title : Pyridostigmine bromide and the long-term subjective health status of a sample of over 700 male Reserve Component Gulf War era veterans - Schumm_2002_Psychol.Rep_90_707 |
| Author(s) : Schumm WR , Reppert EJ , Jurich AP , Bollman SR , Webb FJ , Castelo CS , Stever JC , Kaufman M , Deng LY , Krehbiel M , Owens BL , Hall CA , Brown BF , Lash JF , Fink CJ , Crow JR , Bonjour GN |
| Ref : Psychol Rep , 90 :707 , 2002 |
| Abstract : Schumm_2002_Psychol.Rep_90_707 |
| ESTHER : Schumm_2002_Psychol.Rep_90_707 |
| PubMedSearch : Schumm_2002_Psychol.Rep_90_707 |
| PubMedID: 12090498 |
| Title : Acute and Repeated Restraint Stress Have Little Effect on Pyridostigmine Toxicity or Brain Regional Cholinesterase Inhibition in Rats - Song_2002_Toxicol.Sci_69_157 |
| Author(s) : Song X , Tian H , Bressler J , Pruett S , Pope C |
| Ref : Toxicol Sci , 69 :157 , 2002 |
| Abstract : Song_2002_Toxicol.Sci_69_157 |
| ESTHER : Song_2002_Toxicol.Sci_69_157 |
| PubMedSearch : Song_2002_Toxicol.Sci_69_157 |
| PubMedID: 12215670 |
| Title : Neither forced running nor forced swimming affect acute pyridostigmine toxicity or brain-regional cholinesterase inhibition in rats - Tian_2002_Toxicology_176_39 |
| Author(s) : Tian H , Song X , Bressler J , Pruett S , Pope CN |
| Ref : Toxicology , 176 :39 , 2002 |
| Abstract : Tian_2002_Toxicology_176_39 |
| ESTHER : Tian_2002_Toxicology_176_39 |
| PubMedSearch : Tian_2002_Toxicology_176_39 |
| PubMedID: 12062928 |
| Title : Determination of physostigmine and pyridostigmine in pharmaceutical formulations by capillary electrophoresis - Havel_2002_J.Capill.Electrophor.Microchip.Technol_7_107 |
| Author(s) : Havel J , Patocka J , Bocaz G |
| Ref : J Capill Electrophor Microchip Technol , 7 :107 , 2002 |
| Abstract : Havel_2002_J.Capill.Electrophor.Microchip.Technol_7_107 |
| ESTHER : Havel_2002_J.Capill.Electrophor.Microchip.Technol_7_107 |
| PubMedSearch : Havel_2002_J.Capill.Electrophor.Microchip.Technol_7_107 |
| PubMedID: 12546159 |
| Title : Pyridostigmine bromide modulates the dermal disposition of [14C]permethrin - Baynes_2002_Toxicol.Appl.Pharmacol_181_164 |
| Author(s) : Baynes RE , Monteiro-Riviere NA , Riviere JE |
| Ref : Toxicol Appl Pharmacol , 181 :164 , 2002 |
| Abstract : Baynes_2002_Toxicol.Appl.Pharmacol_181_164 |
| ESTHER : Baynes_2002_Toxicol.Appl.Pharmacol_181_164 |
| PubMedSearch : Baynes_2002_Toxicol.Appl.Pharmacol_181_164 |
| PubMedID: 12079425 |
| Title : Actions of pyridostigmine and organophosphate agents on chick cells, mice, and chickens - Wilson_2002_Drug.Chem.Toxicol_25_131 |
| Author(s) : Wilson BW , Henderson JD , Coatney EM , Nieberg PS , Spencer PS |
| Ref : Drug & Chemical Toxicology , 25 :131 , 2002 |
| Abstract : Wilson_2002_Drug.Chem.Toxicol_25_131 |
| ESTHER : Wilson_2002_Drug.Chem.Toxicol_25_131 |
| PubMedSearch : Wilson_2002_Drug.Chem.Toxicol_25_131 |
| PubMedID: 12024798 |
| Title : Residual paralysis induced by either vecuronium or rocuronium after reversal with pyridostigmine - Kim_2002_Anesth.Analg_95_1656 |
| Author(s) : Kim KS , Lew SH , Cho HY , Cheong MA |
| Ref : Anesthesia & Analgesia , 95 :1656 , 2002 |
| Abstract : Kim_2002_Anesth.Analg_95_1656 |
| ESTHER : Kim_2002_Anesth.Analg_95_1656 |
| PubMedSearch : Kim_2002_Anesth.Analg_95_1656 |
| PubMedID: 12456433 |
| Title : The effect of continuous pyridostigmine administration on functional (A12) acetylcholinesterase activity in guinea-pig muscles - Lintern_2001_Neurotoxicol_22_787 |
| Author(s) : Lintern MC , Wetherell JR , Taylor C , Smith ME |
| Ref : Neurotoxicology , 22 :787 , 2001 |
| Abstract : Lintern_2001_Neurotoxicol_22_787 |
| ESTHER : Lintern_2001_Neurotoxicol_22_787 |
| PubMedSearch : Lintern_2001_Neurotoxicol_22_787 |
| PubMedID: 11829412 |
| Title : Locomotor and sensorimotor performance deficit in rats following exposure to pyridostigmine bromide, DEET, and permethrin, alone and in combination - Abou-Donia_2001_Toxicol.Sci_60_305 |
| Author(s) : Abou-Donia MB , Goldstein LB , Jones KH , Abdel-Rahman AA , Damodaran TV , Dechkovskaia AM , Bullman SL , Amir BE , Khan WA |
| Ref : Toxicol Sci , 60 :305 , 2001 |
| Abstract : Abou-Donia_2001_Toxicol.Sci_60_305 |
| ESTHER : Abou-Donia_2001_Toxicol.Sci_60_305 |
| PubMedSearch : Abou-Donia_2001_Toxicol.Sci_60_305 |
| PubMedID: 11248143 |
| Title : Reactive Oxygen Species Mediate Pyridostigmine-Induced Neuronal Apoptosis: Involvement of Muscarinic and NMDA Receptors - Li_2001_Toxicol.Appl.Pharmacol_177_17 |
| Author(s) : Li L , Shou Y , Borowitz JL , Isom GE |
| Ref : Toxicol Appl Pharmacol , 177 :17 , 2001 |
| Abstract : Li_2001_Toxicol.Appl.Pharmacol_177_17 |
| ESTHER : Li_2001_Toxicol.Appl.Pharmacol_177_17 |
| PubMedSearch : Li_2001_Toxicol.Appl.Pharmacol_177_17 |
| PubMedID: 11708896 |
| Title : Evaluation of immunotoxicity induced by single or concurrent exposure to N,N-diethyl-m-toluamide (DEET), pyridostigmine bromide (PYR), and JP-8 jet fuel - Peden-Adam_2001_Toxicol.Ind.Health_17_192 |
| Author(s) : Peden-Adam MM , Eudaly J , Eudaly E , Dudley A , Zeigler J , Lee A , Robbs J , Gilkeson G , Keil DE |
| Ref : Toxicol Ind Health , 17 :192 , 2001 |
| Abstract : Peden-Adam_2001_Toxicol.Ind.Health_17_192 |
| ESTHER : Peden-Adam_2001_Toxicol.Ind.Health_17_192 |
| PubMedSearch : Peden-Adam_2001_Toxicol.Ind.Health_17_192 |
| PubMedID: 12539864 |
| Title : Enhancement of heart rate variability by cholinergic stimulation with pyridostigmine in healthy subjects - Nobrega_2001_Clin.Auton.Res_11_11 |
| Author(s) : Nobrega AC , dos Reis AF , Moraes RS , Bastos BG , Ferlin EL , Ribeiro JP |
| Ref : Clin Auton Res , 11 :11 , 2001 |
| Abstract : Nobrega_2001_Clin.Auton.Res_11_11 |
| ESTHER : Nobrega_2001_Clin.Auton.Res_11_11 |
| PubMedSearch : Nobrega_2001_Clin.Auton.Res_11_11 |
| PubMedID: 11503945 |
| Title : Pyridostigmine-induced microcephaly - |
| Author(s) : Polizzi A , Ruggieri M , Vincent A |
| Ref : Neurology , 56 :1606 , 2001 |
| PubMedID: 11409415 |
| Title : Side effects of low-dose pyridostigmine bromide are not related to cholinesterase inhibition - Cook_2001_Aviat.Space.Environ.Med_72_1102 |
| Author(s) : Cook MR , Gerkovich MM , Sastre A , Graham C |
| Ref : Aviat Space Environ Med , 72 :1102 , 2001 |
| Abstract : Cook_2001_Aviat.Space.Environ.Med_72_1102 |
| ESTHER : Cook_2001_Aviat.Space.Environ.Med_72_1102 |
| PubMedSearch : Cook_2001_Aviat.Space.Environ.Med_72_1102 |
| PubMedID: 11763111 |
| Title : The combined effects of pyridostigmine and chronic stress on brain cortical and blood acetylcholinesterase, corticosterone, prolactin and alternation performance in rats - Kant_2001_Pharmacol.Biochem.Behav_70_209 |
| Author(s) : Kant GJ , Bauman RA , Feaster SR , Anderson SM , Saviolakis GA , Garcia GE |
| Ref : Pharmacol Biochem Behav , 70 :209 , 2001 |
| Abstract : Kant_2001_Pharmacol.Biochem.Behav_70_209 |
| ESTHER : Kant_2001_Pharmacol.Biochem.Behav_70_209 |
| PubMedSearch : Kant_2001_Pharmacol.Biochem.Behav_70_209 |
| PubMedID: 11701190 |
| Title : Exercise stress testing in healthy subjects during cholinergic stimulation after a single dose of pyridostigmine - Serra_2001_Arq.Bras.Cardiol_76_279 |
| Author(s) : Serra SM , Costa RV , Bastos BG , Bousquet Santos K , Ramalho SH , da Nobrega AC |
| Ref : Arq Bras Cardiol , 76 :279 , 2001 |
| Abstract : Serra_2001_Arq.Bras.Cardiol_76_279 |
| ESTHER : Serra_2001_Arq.Bras.Cardiol_76_279 |
| PubMedSearch : Serra_2001_Arq.Bras.Cardiol_76_279 |
| PubMedID: 11323731 |
| Title : Biological consequences of multiple vaccine and pyridostigmine pretreatment in the guinea pig - Griffiths_2001_J.Appl.Toxicol_21_59 |
| Author(s) : Griffiths GD , Hornby RJ , Stevens DJ , Scott LA , Upshall DG |
| Ref : Journal of Applied Toxicology , 21 :59 , 2001 |
| Abstract : Griffiths_2001_J.Appl.Toxicol_21_59 |
| ESTHER : Griffiths_2001_J.Appl.Toxicol_21_59 |
| PubMedSearch : Griffiths_2001_J.Appl.Toxicol_21_59 |
| PubMedID: 11180281 |
| Title : Pyridostigmine-induced microcephaly - |
| Author(s) : Al-Shekhlee A , Robin N , Kaminski HJ |
| Ref : Neurology , 56 :1606 , 2001 |
| PubMedID: 11402134 |
| Title : Muscarinic receptor-mediated pyridostigmine-induced neuronal apoptosis - Li_2000_Neurotoxicol_21_541 |
| Author(s) : Li L , Gunasekar PG , Borowitz JL , Isom GE |
| Ref : Neurotoxicology , 21 :541 , 2000 |
| Abstract : Li_2000_Neurotoxicol_21_541 |
| ESTHER : Li_2000_Neurotoxicol_21_541 |
| PubMedSearch : Li_2000_Neurotoxicol_21_541 |
| PubMedID: 11022862 |
| Title : Acute effects of an insect repellent, N,N-diethyl-m-toluamide, on cholinesterase inhibition induced by pyridostigmine bromide in rats - Chaney_2000_Toxicol.Appl.Pharmacol_165_107 |
| Author(s) : Chaney LA , Wineman RW , Rockhold RW , Hume AS |
| Ref : Toxicol Appl Pharmacol , 165 :107 , 2000 |
| Abstract : Chaney_2000_Toxicol.Appl.Pharmacol_165_107 |
| ESTHER : Chaney_2000_Toxicol.Appl.Pharmacol_165_107 |
| PubMedSearch : Chaney_2000_Toxicol.Appl.Pharmacol_165_107 |
| PubMedID: 10828206 |
| Title : Interactions of bovine brain tubulin with pyridostigmine bromide and N,N'-diethyl-m-toluamide - Prasad_2000_Neurochem.Res_25_19 |
| Author(s) : Prasad V , Scotch R , Chaudhuri AR , Walss C , Fathy DB , Miller C , Luduena RF |
| Ref : Neurochem Res , 25 :19 , 2000 |
| Abstract : Prasad_2000_Neurochem.Res_25_19 |
| ESTHER : Prasad_2000_Neurochem.Res_25_19 |
| PubMedSearch : Prasad_2000_Neurochem.Res_25_19 |
| PubMedID: 10685600 |
| Title : Interactive and delayed effects of pyridostigmine and physical stress on biochemical and histological changes in peripheral tissues of mice - Somani_2000_J.Appl.Toxicol_20_327 |
| Author(s) : Somani SM , Husain K , Asha T , Helfert R |
| Ref : J Appl Toxicol , 20 :327 , 2000 |
| Abstract : Somani_2000_J.Appl.Toxicol_20_327 |
| ESTHER : Somani_2000_J.Appl.Toxicol_20_327 |
| PubMedSearch : Somani_2000_J.Appl.Toxicol_20_327 |
| PubMedID: 10942908 |
| Title : Stress does not enable pyridostigmine to inhibit brain cholinesterase after parenteral administration - Grauer_2000_Toxicol.Appl.Pharmacol_164_301 |
| Author(s) : Grauer E , Alkalai D , Kapon J , Cohen G , Raveh L |
| Ref : Toxicol Appl Pharmacol , 164 :301 , 2000 |
| Abstract : Grauer_2000_Toxicol.Appl.Pharmacol_164_301 |
| ESTHER : Grauer_2000_Toxicol.Appl.Pharmacol_164_301 |
| PubMedSearch : Grauer_2000_Toxicol.Appl.Pharmacol_164_301 |
| PubMedID: 10799340 |
| Title : Stressful manipulations that elevate corticosterone reduce blood-brain barrier permeability to pyridostigmine in the Rat - Sinton_2000_Toxicol.Appl.Pharmacol_165_99 |
| Author(s) : Sinton CM , Fitch TE , Petty F , Haley RW |
| Ref : Toxicol Appl Pharmacol , 165 :99 , 2000 |
| Abstract : Sinton_2000_Toxicol.Appl.Pharmacol_165_99 |
| ESTHER : Sinton_2000_Toxicol.Appl.Pharmacol_165_99 |
| PubMedSearch : Sinton_2000_Toxicol.Appl.Pharmacol_165_99 |
| PubMedID: 10814558 |
| Title : Effects of inescapable stress and treatment with pyridostigmine bromide on plasma butyrylcholinesterase and the acoustic startle response in rats - Servatius_2000_Physiol.Behav_69_239 |
| Author(s) : Servatius RJ , Ottenweller JE , Guo W , Beldowicz D , Zhu G , Natelson BH |
| Ref : Physiol Behav , 69 :239 , 2000 |
| Abstract : Servatius_2000_Physiol.Behav_69_239 |
| ESTHER : Servatius_2000_Physiol.Behav_69_239 |
| PubMedSearch : Servatius_2000_Physiol.Behav_69_239 |
| PubMedID: 10869589 |
| Title : Delayed effects of pyridostigmine and exercise training on acetylcholinesterase and muscle tension in mouse lower extremity - Verma-Ahuja_2000_Arch.Toxicol_74_539 |
| Author(s) : Verma-Ahuja S , Husain K , Verhulst S , Espinosa JA , Somani SM |
| Ref : Archives of Toxicology , 74 :539 , 2000 |
| Abstract : Verma-Ahuja_2000_Arch.Toxicol_74_539 |
| ESTHER : Verma-Ahuja_2000_Arch.Toxicol_74_539 |
| PubMedSearch : Verma-Ahuja_2000_Arch.Toxicol_74_539 |
| PubMedID: 11131034 |
| Title : Effects of exposure to low-dose pyridostigmine on neuromuscular junctions in vitro - Drake-Baumann_1999_Muscle.Nerve_22_696 |
| Author(s) : Drake-Baumann R , Seil FJ |
| Ref : Muscle & Nerve , 22 :696 , 1999 |
| Abstract : Drake-Baumann_1999_Muscle.Nerve_22_696 |
| ESTHER : Drake-Baumann_1999_Muscle.Nerve_22_696 |
| PubMedSearch : Drake-Baumann_1999_Muscle.Nerve_22_696 |
| PubMedID: 10366222 |
| Title : Pyridostigmine, a carbamate acetylcholinesterase AChE inhibitor and reactivator, is used prophylactically against chemical warfare agents [letter] - Telang_1999_Nucl.Med.Biol_26_249 |
| Author(s) : Telang FW , Ding YS , Volkow ND , Molina PE , Gatley SJ |
| Ref : Nucl Med Biol , 26 :249 , 1999 |
| Abstract : Telang_1999_Nucl.Med.Biol_26_249 |
| ESTHER : Telang_1999_Nucl.Med.Biol_26_249 |
| PubMedSearch : Telang_1999_Nucl.Med.Biol_26_249 |
| PubMedID: 10100227 |
| Title : GH response to GHRH combined with pyridostigmine or arginine in different conditions of low somatotrope secretion in adulthood: obesity and Cushing's syndrome in comparison with hypopituitarism - Procopio_1999_Minerva.Endocrinol_24_107 |
| Author(s) : Procopio M , Maccario M , Savio P , Valetto MR , Aimaretti G , Grottoli S , Oleandri SE , Baffoni C , Tassone F , Arvat E , Camanni F , Ghigo E |
| Ref : Minerva Endocrinol , 24 :107 , 1999 |
| Abstract : Procopio_1999_Minerva.Endocrinol_24_107 |
| ESTHER : Procopio_1999_Minerva.Endocrinol_24_107 |
| PubMedSearch : Procopio_1999_Minerva.Endocrinol_24_107 |
| PubMedID: 10953725 |
| Title : Success of pyridostigmine, physostigmine, eptastigmine and phosphotriesterase treatments in acute sarin intoxication - Tuovinen_1999_Toxicology_134_169 |
| Author(s) : Tuovinen K , Kaliste-Korhonen E , Raushel FM , Hanninen O |
| Ref : Toxicology , 134 :169 , 1999 |
| Abstract : Tuovinen_1999_Toxicology_134_169 |
| ESTHER : Tuovinen_1999_Toxicology_134_169 |
| PubMedSearch : Tuovinen_1999_Toxicology_134_169 |
| PubMedID: 10403635 |
| Title : Pyridostigmine cotreatment for controlled ovarian hyperstimulation in low responders undergoing in vitro fertilization-embryo transfer - Kim_1999_Fertil.Steril_71_652 |
| Author(s) : Kim CH , Chae HD , Chang YS |
| Ref : Fertil Steril , 71 :652 , 1999 |
| Abstract : Kim_1999_Fertil.Steril_71_652 |
| ESTHER : Kim_1999_Fertil.Steril_71_652 |
| PubMedSearch : Kim_1999_Fertil.Steril_71_652 |
| PubMedID: 10202874 |
| Title : Population pharmacokinetics and pharmacodynamics of pyridostigmine bromide for prophylaxis against nerve agents in humans - Marino_1998_J.Clin.Pharmacol_38_227 |
| Author(s) : Marino MT , Schuster BG , Brueckner RP , Lin E , Kaminskis A , Lasseter KC |
| Ref : Journal of Clinical Pharmacology , 38 :227 , 1998 |
| Abstract : Marino_1998_J.Clin.Pharmacol_38_227 |
| ESTHER : Marino_1998_J.Clin.Pharmacol_38_227 |
| PubMedSearch : Marino_1998_J.Clin.Pharmacol_38_227 |
| PubMedID: 9549661 |
| Title : Heat stress, even extreme, does not induce penetration of pyridostigmine into the brain of guinea-pigs - |
| Author(s) : Lallement G , Foquin A , Baubichon D , Burckhart MF , Carpentier P , Canini F |
| Ref : Journal de Physiologie (Paris) , 92 :453 , 1998 |
| PubMedID: |
| Title : Heat stress, even extreme, does not induce penetration of pyridostigmine into the brain of guinea pigs - Lallement_1998_Neurotoxicol_19_759 |
| Author(s) : Lallement G , Foquin A , Baubichon D , Burckhart MF , Carpentier P , Canini F |
| Ref : Neurotoxicology , 19 :759 , 1998 |
| Abstract : Lallement_1998_Neurotoxicol_19_759 |
| ESTHER : Lallement_1998_Neurotoxicol_19_759 |
| PubMedSearch : Lallement_1998_Neurotoxicol_19_759 |
| PubMedID: 9863765 |
| Title : Pyridostigmine bromide and Gulf War syndrome - Shen_1998_Med.Hypotheses_51_235 |
| Author(s) : Shen ZX |
| Ref : Med Hypotheses , 51 :235 , 1998 |
| Abstract : Shen_1998_Med.Hypotheses_51_235 |
| ESTHER : Shen_1998_Med.Hypotheses_51_235 |
| PubMedSearch : Shen_1998_Med.Hypotheses_51_235 |
| PubMedID: 9792201 |
| Title : A Comparison of Blood Cholinesterase Activities, Pyridostigmine Inhibition of Red Cell Acetylcholinesterase, and Butyrylcholinesterase Phenotypes in Gulf War Veterans and Normal Controls - |
| Author(s) : Gentry MK , Powell S , Bitsko N , Bartels CF , Bartko J , Chung R , Lockridge O , Ribas J , Roy M , Malone J , Doctor BP |
| Ref : In: Structure and Function of Cholinesterases and Related Proteins - Proceedings of Sixth International Meeting on Cholinesterases , (Doctor, B.P., Taylor, P., Quinn, D.M., Rotundo, R.L., Gentry, M.K. Eds) Plenum Publishing Corp. :300 , 1998 |
| PubMedID: |
| Title : Plasma GH responses to GHRH, arginine, L-dopa, pyridostigmine, sequential administrations of GHRH and combined administration of PD and GHRH in Turner's syndrome - Hanew_1998_J.Endocrinol.Invest_21_72 |
| Author(s) : Hanew K , Tanaka A , Utsumi A |
| Ref : J Endocrinol Invest , 21 :72 , 1998 |
| Abstract : Hanew_1998_J.Endocrinol.Invest_21_72 |
| ESTHER : Hanew_1998_J.Endocrinol.Invest_21_72 |
| PubMedSearch : Hanew_1998_J.Endocrinol.Invest_21_72 |
| PubMedID: 9585379 |
| Title : Persistently exaggerated startle responses in rats treated with pyridostigmine bromide - Servatius_1998_J.Pharmacol.Exp.Ther_287_1020 |
| Author(s) : Servatius RJ , Ottenweller JE , Beldowicz D , Guo W , Zhu G , Natelson BH |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 287 :1020 , 1998 |
| Abstract : Servatius_1998_J.Pharmacol.Exp.Ther_287_1020 |
| ESTHER : Servatius_1998_J.Pharmacol.Exp.Ther_287_1020 |
| PubMedSearch : Servatius_1998_J.Pharmacol.Exp.Ther_287_1020 |
| PubMedID: 9864288 |
| Title : Different effects of pyridostigmine on the thyrotropin response to thyrotropin-releasing hormone in endogenous depression and subclinical thyrotoxicosis - Coiro_1998_Metabolism_47_50 |
| Author(s) : Coiro V , Volpi R , Marchesi C , DeFerri A , Capretti L , Caffarri G , Colla R , Chiodera P |
| Ref : Metabolism , 47 :50 , 1998 |
| Abstract : Coiro_1998_Metabolism_47_50 |
| ESTHER : Coiro_1998_Metabolism_47_50 |
| PubMedSearch : Coiro_1998_Metabolism_47_50 |
| PubMedID: 9440477 |
| Title : Mapping of cerebral metabolic activation in three models of cholinergic convulsions - Scremin_1998_Brain.Res.Bull_45_167 |
| Author(s) : Scremin OU , Shih TM , Li MG , Jenden DJ |
| Ref : Brain Research Bulletin , 45 :167 , 1998 |
| Abstract : Scremin_1998_Brain.Res.Bull_45_167 |
| ESTHER : Scremin_1998_Brain.Res.Bull_45_167 |
| PubMedSearch : Scremin_1998_Brain.Res.Bull_45_167 |
| PubMedID: 9443835 |
| Title : GH response to GHRH combined with pyridostigmine or arginine in different conditions of low somatotrope secretion in adulthood: obesity and Cushing's syndrome in comparison with hypopituitarism - Procopio_1998_Panminerva.Med_40_13 |
| Author(s) : Procopio M , Maccario M , Savio P , Valetto MR , Aimaretti G , Grottoli S , Oleandri SE , Baffoni C , Tassone F , Arvat E , Camanni F , Ghigo E |
| Ref : Panminerva Med , 40 :13 , 1998 |
| Abstract : Procopio_1998_Panminerva.Med_40_13 |
| ESTHER : Procopio_1998_Panminerva.Med_40_13 |
| PubMedSearch : Procopio_1998_Panminerva.Med_40_13 |
| PubMedID: 9573747 |
| Title : Reduced growth hormone (GH) responsiveness to combined GH-releasing hormone and pyridostigmine administration in the Prader-Willi syndrome - Grugni_1998_Clin.Endocrinol.(Oxf)_48_769 |
| Author(s) : Grugni G , Guzzaloni G , Moro D , Bettio D , De Medici C , Morabito F |
| Ref : Clinical Endocrinology (Oxf) , 48 :769 , 1998 |
| Abstract : Grugni_1998_Clin.Endocrinol.(Oxf)_48_769 |
| ESTHER : Grugni_1998_Clin.Endocrinol.(Oxf)_48_769 |
| PubMedSearch : Grugni_1998_Clin.Endocrinol.(Oxf)_48_769 |
| PubMedID: 9713567 |
| Title : Effect of pyridostigmine administration in mice on the expression of motoneuron beta-endorphin and muscle acetylcholinesterase - |
| Author(s) : Smith ME , Amos ML , Lintern MC |
| Ref : Journal de Physiologie (Paris) , 92 :495 , 1998 |
| PubMedID: |
| Title : Effect of continuous administration of pyridostigmine on the activity of functional acetylcholinesterase in guinea-pig muscle and brain - |
| Author(s) : Lintern MC , Wetherell J , Smith ME |
| Ref : Journal de Physiologie (Paris) , 92 :459 , 1998 |
| PubMedID: |
| Title : Repeated administration of growth hormone-releasing hormone with or without previous administration of pyridostigmine in insulin-dependent diabetes mellitus - Martina_1997_Horm.Metab.Res_29_180 |
| Author(s) : Martina V , Bruno G , Tagliabue M , Maccario M , Bertaina S , Zumpano E , Arvat E , Ghigo E , Camanni F |
| Ref : Hormone & Metabolic Research , 29 :180 , 1997 |
| Abstract : Martina_1997_Horm.Metab.Res_29_180 |
| ESTHER : Martina_1997_Horm.Metab.Res_29_180 |
| PubMedSearch : Martina_1997_Horm.Metab.Res_29_180 |
| PubMedID: 9178028 |
| Title : Effect of pyridostigmine on the thyroid-stimulating hormone response to thyrotropin-releasing hormone in abstinent alcoholics - Coiro_1997_Alcohol.Clin.Exp.Res_21_1308 |
| Author(s) : Coiro V , Vescovi PP |
| Ref : Alcohol Clin Exp Res , 21 :1308 , 1997 |
| Abstract : Coiro_1997_Alcohol.Clin.Exp.Res_21_1308 |
| ESTHER : Coiro_1997_Alcohol.Clin.Exp.Res_21_1308 |
| PubMedSearch : Coiro_1997_Alcohol.Clin.Exp.Res_21_1308 |
| PubMedID: 9347094 |
| Title : Cholinergic enhancement by pyridostigmine increases the insulin response to glucose load in obese patients but not in normal subjects - Del Rio_1997_Int.J.Obes.Relat.Metab.Disord_21_1111 |
| Author(s) : Del Rio G , Procopio M , Bondi M , Marrama P , Menozzi R , Oleandri SE , Grottoli S , Maccario M , Velardo A , Ghigo E |
| Ref : Int J Obes Relat Metab Disord , 21 :1111 , 1997 |
| Abstract : Del Rio_1997_Int.J.Obes.Relat.Metab.Disord_21_1111 |
| ESTHER : Del Rio_1997_Int.J.Obes.Relat.Metab.Disord_21_1111 |
| PubMedSearch : Del Rio_1997_Int.J.Obes.Relat.Metab.Disord_21_1111 |
| PubMedID: 9426377 |
| Title : Pyridostigmine treatment selectively amplifies the mass of GH secreted per burst without altering GH burst frequency, half-life, basal GH secretion or the orderliness of GH release[see comments] - Friend_1997_Eur.J.Endocrinol_137_377 |
| Author(s) : Friend K , Iranmanesh A , Login IS , Veldhuis JD |
| Ref : European Journal of Endocrinology , 137 :377 , 1997 |
| Abstract : Friend_1997_Eur.J.Endocrinol_137_377 |
| ESTHER : Friend_1997_Eur.J.Endocrinol_137_377 |
| PubMedSearch : Friend_1997_Eur.J.Endocrinol_137_377 |
| PubMedID: 9368506 |
| Title : Lack of bioavailability of mebeverine even after pretreatment with pyridostigmine - Sommers_1997_Eur.J.Clin.Pharmacol_53_247 |
| Author(s) : Sommers DK , Snyman JR , van Wyk M , Eloff JN |
| Ref : European Journal of Clinical Pharmacology , 53 :247 , 1997 |
| Abstract : Sommers_1997_Eur.J.Clin.Pharmacol_53_247 |
| ESTHER : Sommers_1997_Eur.J.Clin.Pharmacol_53_247 |
| PubMedSearch : Sommers_1997_Eur.J.Clin.Pharmacol_53_247 |
| PubMedID: 9476039 |
| Title : Specificity of the pyridostigmine\/growth hormone challenge in the diagnosis of depression - Cooney_1997_Biol.Psychiatry_42_827 |
| Author(s) : Cooney JM , Lucey JV , O'Keane V , Dinan TG |
| Ref : Biological Psychiatry , 42 :827 , 1997 |
| Abstract : Cooney_1997_Biol.Psychiatry_42_827 |
| ESTHER : Cooney_1997_Biol.Psychiatry_42_827 |
| PubMedSearch : Cooney_1997_Biol.Psychiatry_42_827 |
| PubMedID: 9347132 |
| Title : Bradycardia produced by pyridostigmine and physostigmine - Stein_1997_Can.J.Anaesth_44_1286 |
| Author(s) : Stein RD , Backman SB , Collier B , Polosa C |
| Ref : Canadian Journal of Anaesthesia , 44 :1286 , 1997 |
| Abstract : Stein_1997_Can.J.Anaesth_44_1286 |
| ESTHER : Stein_1997_Can.J.Anaesth_44_1286 |
| PubMedSearch : Stein_1997_Can.J.Anaesth_44_1286 |
| PubMedID: 9429048 |
| Title : Potentiation of pyridostigmine bromide toxicity in mice by selected adrenergic agents and caffeine - Chaney_1997_Vet.Hum.Toxicol_39_214 |
| Author(s) : Chaney LA , Rockhold RW , Mozingo JR , Hume AS , Moss JI |
| Ref : Vet Hum Toxicol , 39 :214 , 1997 |
| Abstract : Chaney_1997_Vet.Hum.Toxicol_39_214 |
| ESTHER : Chaney_1997_Vet.Hum.Toxicol_39_214 |
| PubMedSearch : Chaney_1997_Vet.Hum.Toxicol_39_214 |
| PubMedID: 9251170 |
| Title : Low-dose guanidine and pyridostigmine: relatively safe and effective long-term symptomatic therapy in Lambert-Eaton myasthenic syndrome - Oh_1997_Muscle.Nerve_20_1146 |
| Author(s) : Oh SJ , Kim DS , Head TC , Claussen GC |
| Ref : Muscle & Nerve , 20 :1146 , 1997 |
| Abstract : Oh_1997_Muscle.Nerve_20_1146 |
| ESTHER : Oh_1997_Muscle.Nerve_20_1146 |
| PubMedSearch : Oh_1997_Muscle.Nerve_20_1146 |
| PubMedID: 9270671 |
| Title : Effects of pyridostigmine on acetylcholinesterase in different muscles of the mouse - Lintern_1997_Human.Exp.Toxicol_16_18 |
| Author(s) : Lintern MC , Smith ME , Ferry CB |
| Ref : Human & Experimental Toxicology , 16 :18 , 1997 |
| Abstract : Lintern_1997_Human.Exp.Toxicol_16_18 |
| ESTHER : Lintern_1997_Human.Exp.Toxicol_16_18 |
| PubMedSearch : Lintern_1997_Human.Exp.Toxicol_16_18 |
| PubMedID: 9023571 |
| Title : Low hexarelin dose and pyridostigmine have additive effect and potentiate to the same extent the GHRH-induced GH response in man - Arvat_1997_Clin.Endocrinol.(Oxf)_47_495 |
| Author(s) : Arvat E , Di Vito L , Ramunni J , Gianotti L , Giordano R , Deghenghi R , Camanni F , Ghigo E |
| Ref : Clinical Endocrinology (Oxf) , 47 :495 , 1997 |
| Abstract : Arvat_1997_Clin.Endocrinol.(Oxf)_47_495 |
| ESTHER : Arvat_1997_Clin.Endocrinol.(Oxf)_47_495 |
| PubMedSearch : Arvat_1997_Clin.Endocrinol.(Oxf)_47_495 |
| PubMedID: 9404449 |
| Title : Neurotoxicity resulting from coexposure to pyridostigmine bromide, deet, and permethrin: implications of Gulf War chemical exposures - Abou-Donia_1996_J.Toxicol.Env.Health_48_35 |
| Author(s) : Abou-Donia MB , Wilmarth KR , Jensen KF , Oehme FW , Kurt TL |
| Ref : Journal of Toxicology & Environmental Health , 48 :35 , 1996 |
| Abstract : Abou-Donia_1996_J.Toxicol.Env.Health_48_35 |
| ESTHER : Abou-Donia_1996_J.Toxicol.Env.Health_48_35 |
| PubMedSearch : Abou-Donia_1996_J.Toxicol.Env.Health_48_35 |
| PubMedID: 8637057 |
| Title : Acute administration of human galanin in normal subjects reduces the potentiating effect of pyridostigmine-induced cholinergic enhancement on release of norepinephrine and pancreatic polypeptide - degli_1996_Neuroendocrinology_64_398 |
| Author(s) : degli Uberti EC , Bondanelli M , Margutti A , Ambrosio MR , Valentini A , Campo M , Franceschetti P , Zatelli MC , Pansini R , Trasforini G |
| Ref : Neuroendocrinology , 64 :398 , 1996 |
| Abstract : degli_1996_Neuroendocrinology_64_398 |
| ESTHER : degli_1996_Neuroendocrinology_64_398 |
| PubMedSearch : degli_1996_Neuroendocrinology_64_398 |
| PubMedID: 8930940 |
| Title : Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response [see comments] - Friedman_1996_Nature.Med_2_1382 |
| Author(s) : Friedman A , Kaufer D , Shemer J , Hendler I , Soreq H , Tur-Kaspa I |
| Ref : Nature Medicine , 2 :1382 , 1996 |
| Abstract : Friedman_1996_Nature.Med_2_1382 |
| ESTHER : Friedman_1996_Nature.Med_2_1382 |
| PubMedSearch : Friedman_1996_Nature.Med_2_1382 |
| PubMedID: 8946841 |
| Title : Neuromuscular blocking action of suxamethonium after antagonism of vecuronium by edrophonium, pyridostigmine or neostigmine - Fleming_1996_Br.J.Anaesth_77_492 |
| Author(s) : Fleming NW , Macres S , Antognini JF , Vengco J |
| Ref : British Journal of Anaesthesia , 77 :492 , 1996 |
| Abstract : Fleming_1996_Br.J.Anaesth_77_492 |
| ESTHER : Fleming_1996_Br.J.Anaesth_77_492 |
| PubMedSearch : Fleming_1996_Br.J.Anaesth_77_492 |
| PubMedID: 8942334 |
| Title : The effects of nerve agent pre-treatment with pyridostigmine on the duration of action of suxamethonium [letter] - |
| Author(s) : Heath KJ , Niemiro LA , Gosden EA , Restall J |
| Ref : Anaesthesia , 51 :404 , 1996 |
| PubMedID: 8686841 |
| Title : Anticholinesterase drugs stimulate phosphatidylinositol response in rat tracheal slices - Shibata_1996_Anesth.Analg_82_1211 |
| Author(s) : Shibata O , Kanairo M , Zhang S , Hasuo H , Morooka H , Fujie T , Sumikawa K |
| Ref : Anesthesia & Analgesia , 82 :1211 , 1996 |
| Abstract : Shibata_1996_Anesth.Analg_82_1211 |
| ESTHER : Shibata_1996_Anesth.Analg_82_1211 |
| PubMedSearch : Shibata_1996_Anesth.Analg_82_1211 |
| PubMedID: 8638793 |
| Title : The effect of ondansetron on pyridostigmine-induced blood acetylcholinesterase inhibition in the guinea pig - Capacio_1996_Drug.Chem.Toxicol_19_1 |
| Author(s) : Capacio BR , Byers CE , Anderson DR , Matthews RL , Brown DE |
| Ref : Drug & Chemical Toxicology , 19 :1 , 1996 |
| Abstract : Capacio_1996_Drug.Chem.Toxicol_19_1 |
| ESTHER : Capacio_1996_Drug.Chem.Toxicol_19_1 |
| PubMedSearch : Capacio_1996_Drug.Chem.Toxicol_19_1 |
| PubMedID: 8804550 |
| Title : Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos - Abou-Donia_1996_Fundam.Appl.Toxicol_34_201 |
| Author(s) : Abou-Donia MB , Wilmarth KR , Abdel-Rahman AA , Jensen KF , Oehme FW , Kurt TL |
| Ref : Fundamental & Applied Toxicology , 34 :201 , 1996 |
| Abstract : Abou-Donia_1996_Fundam.Appl.Toxicol_34_201 |
| ESTHER : Abou-Donia_1996_Fundam.Appl.Toxicol_34_201 |
| PubMedSearch : Abou-Donia_1996_Fundam.Appl.Toxicol_34_201 |
| PubMedID: 8954750 |
| Title : Ocular effects of hyoscine in double dose transdermal administration and its reversal by low dose pyridostigmine - Alhalel_1995_Aviat.Space.Environ.Med_66_1037 |
| Author(s) : Alhalel A , Ziv I , Versano D , Ruach M , Alkalay M , Almog S , Izraeli S , Glovinsky J |
| Ref : Aviat Space Environ Med , 66 :1037 , 1995 |
| Abstract : Alhalel_1995_Aviat.Space.Environ.Med_66_1037 |
| ESTHER : Alhalel_1995_Aviat.Space.Environ.Med_66_1037 |
| PubMedSearch : Alhalel_1995_Aviat.Space.Environ.Med_66_1037 |
| PubMedID: 96126519 |
| Title : Comparative effects of plasma exchange and pyridostigmine on respiratory muscle strength and breathing pattern in patients with myasthenia gravis - Goti_1995_Thorax_50_1080 |
| Author(s) : Goti P , Spinelli A , Marconi G , Duranti R , Gigliotti F , Pizzi A , Scano G |
| Ref : Thorax , 50 :1080 , 1995 |
| Abstract : Goti_1995_Thorax_50_1080 |
| ESTHER : Goti_1995_Thorax_50_1080 |
| PubMedSearch : Goti_1995_Thorax_50_1080 |
| PubMedID: 7491557 |
| Title : Comparative pharmacokinetics of four cholinesterase inhibitors in rats - Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| Author(s) : Yamamoto K , Sawada Y , Iga T |
| Ref : Biol Pharm Bull , 18 :1292 , 1995 |
| Abstract : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| ESTHER : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| PubMedSearch : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| PubMedID: 8845827 |
| Title : Oxime effects on the rate constants of carbamylation and decarbamylation of acetylcholinesterase for pyridostigmine, physostigmine and insecticidal carbamates - Dawson_1995_Neurochem.Int_26_643 |
| Author(s) : Dawson RM |
| Ref : Neurochem Int , 26 :643 , 1995 |
| Abstract : Dawson_1995_Neurochem.Int_26_643 |
| ESTHER : Dawson_1995_Neurochem.Int_26_643 |
| PubMedSearch : Dawson_1995_Neurochem.Int_26_643 |
| PubMedID: 7670367 |
| Title : Comparison of Acetylcholinesterase, Pyridostigmine, and HI-6 as Antidotes against Organophosphorus Compounds - |
| Author(s) : Maxwell DM , Brecht KM , Saxena A , Taylor P , Doctor BP |
| Ref : In Enzyme of the Cholinesterase Family - Proceedings of Fifth International Meeting on Cholinesterases , (Quinn, D.M., Balasubramanian, A.S., Doctor, B.P., Taylor, P., Eds) Plenum Publishing Corp. :353 , 1995 |
| PubMedID: |
| Title : Aging alters the pharmacokinetics of pyridostigmine - Stone_1995_Anesth.Analg_81_773 |
| Author(s) : Stone JG , Matteo RS , Ornstein E , Schwartz AE , Ostapkovich N , Jamdar SC , Diaz J |
| Ref : Anesthesia & Analgesia , 81 :773 , 1995 |
| Abstract : Stone_1995_Anesth.Analg_81_773 |
| ESTHER : Stone_1995_Anesth.Analg_81_773 |
| PubMedSearch : Stone_1995_Anesth.Analg_81_773 |
| PubMedID: 7574009 |
| Title : Protective Efficacy of Physostigmine and Pyridostigmine at Various Pretreatment Times against Inhaled Sarin in Rats - |
| Author(s) : Vijayaraghavan R , Husain K , Kumar P , Pandey KS , Das Gupta S |
| Ref : In Enzyme of the Cholinesterase Family - Proceedings of Fifth International Meeting on Cholinesterases , (Quinn, D.M., Balasubramanian, A.S., Doctor, B.P., Taylor, P., Eds) Plenum Publishing Corp. :408 , 1995 |
| PubMedID: |
| Title : Combined pyridostigmine-thyrotrophin-releasing hormone test for the evaluation of hypothalamic somatostatinergic activity in healthy normal men - Yang_1995_Eur.J.Endocrinol_133_457 |
| Author(s) : Yang I , Woo J , Kim S , Kim J , Kim Y , Choi Y |
| Ref : European Journal of Endocrinology , 133 :457 , 1995 |
| Abstract : Yang_1995_Eur.J.Endocrinol_133_457 |
| ESTHER : Yang_1995_Eur.J.Endocrinol_133_457 |
| PubMedSearch : Yang_1995_Eur.J.Endocrinol_133_457 |
| PubMedID: 7581970 |
| Title : Cardiorespiratory function in nerve agent poisoned and oxime + atropine treated guinea-pigs: effect of pyridostigmine pretreatment - Worek_1995_Arch.Toxicol_69_322 |
| Author(s) : Worek F , Szinicz L |
| Ref : Archives of Toxicology , 69 :322 , 1995 |
| Abstract : Worek_1995_Arch.Toxicol_69_322 |
| ESTHER : Worek_1995_Arch.Toxicol_69_322 |
| PubMedSearch : Worek_1995_Arch.Toxicol_69_322 |
| PubMedID: 7654137 |
| Title : Effect of pyridostigmine pretreatment on cardiorespiratory function in tabun poisoning - Worek_1995_Human.Exp.Toxicol_14_634 |
| Author(s) : Worek F , Kleine A , Szinicz L |
| Ref : Hum Exp Toxicol , 14 :634 , 1995 |
| Abstract : Worek_1995_Human.Exp.Toxicol_14_634 |
| ESTHER : Worek_1995_Human.Exp.Toxicol_14_634 |
| PubMedSearch : Worek_1995_Human.Exp.Toxicol_14_634 |
| PubMedID: 7576830 |
| Title : Acute behavioral toxicity of pyridostigmine or soman in primates - Blick_1994_Toxicol.Appl.Pharmacol_126_311 |
| Author(s) : Blick DW , Murphy MR , Brown GC , Yochmowitz MG , Fanton JW , Hartgraves SL |
| Ref : Toxicology & Applied Pharmacology , 126 :311 , 1994 |
| Abstract : Blick_1994_Toxicol.Appl.Pharmacol_126_311 |
| ESTHER : Blick_1994_Toxicol.Appl.Pharmacol_126_311 |
| PubMedSearch : Blick_1994_Toxicol.Appl.Pharmacol_126_311 |
| PubMedID: 8209384 |
| Title : Primate performance decrements following acute soman exposure: failure of chemical countermeasures - Blick_1994_Pharmacol.Biochem.Behav_49_503 |
| Author(s) : Blick DW , Murphy MR , Brown GC , Hartgraves SL |
| Ref : Pharmacology, Biochemistry & Behavior , 49 :503 , 1994 |
| Abstract : Blick_1994_Pharmacol.Biochem.Behav_49_503 |
| ESTHER : Blick_1994_Pharmacol.Biochem.Behav_49_503 |
| PubMedSearch : Blick_1994_Pharmacol.Biochem.Behav_49_503 |
| PubMedID: 7862701 |
| Title : Pyridostigmine bromide does not alter thermoregulation during exercise in cold air - Roberts_1994_Can.J.Physiol.Pharmacol_72_788 |
| Author(s) : Roberts DE , Sawka MN , Young AJ , Freund BJ |
| Ref : Canadian Journal of Physiology & Pharmacology , 72 :788 , 1994 |
| Abstract : Roberts_1994_Can.J.Physiol.Pharmacol_72_788 |
| ESTHER : Roberts_1994_Can.J.Physiol.Pharmacol_72_788 |
| PubMedSearch : Roberts_1994_Can.J.Physiol.Pharmacol_72_788 |
| PubMedID: 7828087 |
| Title : Comparison of antidote protection against soman by pyridostigmine, HI-6 and acetylcholinesterase - Maxwell_1993_J.Pharmacol.Exp.Ther_264_1085 |
| Author(s) : Maxwell DM , Brecht KM , Doctor BP , Wolfe AD |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 264 :1085 , 1993 |
| Abstract : Maxwell_1993_J.Pharmacol.Exp.Ther_264_1085 |
| ESTHER : Maxwell_1993_J.Pharmacol.Exp.Ther_264_1085 |
| PubMedSearch : Maxwell_1993_J.Pharmacol.Exp.Ther_264_1085 |
| PubMedID: 8450452 |
| Title : Effect of pyridostigmine pretreatment, HI-6 and Toxogonin treatment on rat tracheal smooth muscle response to cholinergic stimulation after organophosphorus inhalation exposure - Walday_1993_Arch.Toxicol_67_212 |
| Author(s) : Walday P , Aas P , Haider T , Fonnum F |
| Ref : Archives of Toxicology , 67 :212 , 1993 |
| Abstract : Walday_1993_Arch.Toxicol_67_212 |
| ESTHER : Walday_1993_Arch.Toxicol_67_212 |
| PubMedSearch : Walday_1993_Arch.Toxicol_67_212 |
| PubMedID: 8494501 |
| Title : Seven-day pyridostigmine administration and thermoregulation during rest and exercise in dry heat - Wenger_1993_Aviat.Space.Environ.Med_64_905 |
| Author(s) : Wenger B , Quigley MD , Kolka MA |
| Ref : Aviat Space Environ Med , 64 :905 , 1993 |
| Abstract : Wenger_1993_Aviat.Space.Environ.Med_64_905 |
| ESTHER : Wenger_1993_Aviat.Space.Environ.Med_64_905 |
| PubMedSearch : Wenger_1993_Aviat.Space.Environ.Med_64_905 |
| PubMedID: 8240194 |
| Title : Reduction by pyridostigmine pretreatment of the efficacy of atropine and 2-PAM treatment of sarin and VX poisoning in rodents - Koplovitz_1992_Fundam.Appl.Toxicol_18_102 |
| Author(s) : Koplovitz I , Harris LW , Anderson DR , Lennox WJ , Stewart JR |
| Ref : Fundamental & Applied Toxicology , 18 :102 , 1992 |
| Abstract : Koplovitz_1992_Fundam.Appl.Toxicol_18_102 |
| ESTHER : Koplovitz_1992_Fundam.Appl.Toxicol_18_102 |
| PubMedSearch : Koplovitz_1992_Fundam.Appl.Toxicol_18_102 |
| PubMedID: 160120 |
| Title : The effect of pyridostigmine pretreatment on oxime efficacy against intoxication by soman or VX in rats - Anderson_1992_Drug.Chem.Toxicol_15_285 |
| Author(s) : Anderson DR , Harris LW , Woodard CL , Lennox WJ |
| Ref : Drug & Chemical Toxicology , 15 :285 , 1992 |
| Abstract : Anderson_1992_Drug.Chem.Toxicol_15_285 |
| ESTHER : Anderson_1992_Drug.Chem.Toxicol_15_285 |
| PubMedSearch : Anderson_1992_Drug.Chem.Toxicol_15_285 |
| PubMedID: 1459041 |
| Title : Effects of subacute pyridostigmine administration on mammalian skeletal muscle function - Adler_1992_J.Appl.Toxicol_12_25 |
| Author(s) : Adler M , Deshpande SS , Foster RE , Maxwell DM , Albuquerque EX |
| Ref : J Appl Toxicol , 12 :25 , 1992 |
| Abstract : Adler_1992_J.Appl.Toxicol_12_25 |
| ESTHER : Adler_1992_J.Appl.Toxicol_12_25 |
| PubMedSearch : Adler_1992_J.Appl.Toxicol_12_25 |
| PubMedID: 1564249 |
| Title : Acute effects of oral pyridostigmine bromide on conditioned operant performance in rats - Shih_1991_Pharmacol.Biochem.Behav_38_549 |
| Author(s) : Shih JH , Liu WF , Lee SF , Lee JD , Ma C , Lin CH |
| Ref : Pharmacol Biochem Behav , 38 :549 , 1991 |
| Abstract : Shih_1991_Pharmacol.Biochem.Behav_38_549 |
| ESTHER : Shih_1991_Pharmacol.Biochem.Behav_38_549 |
| PubMedSearch : Shih_1991_Pharmacol.Biochem.Behav_38_549 |
| PubMedID: 2068191 |
| Title : Effects of subacute pretreatment with carbamate together with acute adjunct pretreatment against nerve agent exposure - Anderson_1991_Drug.Chem.Toxicol_14_1 |
| Author(s) : Anderson DR , Harris LW , Lennox WJ , Solana RP |
| Ref : Drug & Chemical Toxicology , 14 :1 , 1991 |
| Abstract : Anderson_1991_Drug.Chem.Toxicol_14_1 |
| ESTHER : Anderson_1991_Drug.Chem.Toxicol_14_1 |
| PubMedSearch : Anderson_1991_Drug.Chem.Toxicol_14_1 |
| PubMedID: 1889370 |
| Title : A novel tertiary pyridostigmine derivative [3-(N,N-dimethylcarbamyloxy)-1-methyl-delta 3-tetrahydropyridine]: anticholinesterase properties and efficacy against soman - Ray_1991_Fundam.Appl.Toxicol_16_267 |
| Author(s) : Ray R , Clark OEd , Ford KW , Knight KR , Harris LW , Broomfield CA |
| Ref : Fundamental & Applied Toxicology , 16 :267 , 1991 |
| Abstract : Ray_1991_Fundam.Appl.Toxicol_16_267 |
| ESTHER : Ray_1991_Fundam.Appl.Toxicol_16_267 |
| PubMedSearch : Ray_1991_Fundam.Appl.Toxicol_16_267 |
| PubMedID: 2055358 |
| Title : Reversal of cholinesterase inhibition and clinical signs and the postmortem findings in mice after intraperitoneal administration of anatoxin-a(s), paraoxon or pyridostigmine - Cook_1991_Vet.Hum.Toxicol_33_1 |
| Author(s) : Cook WO , Dahlem AM , Harlin KS , Beasley VR , Hooser SB , Haschek WM , Carmicheal WW |
| Ref : Vet Hum Toxicol , 33 :1 , 1991 |
| Abstract : Cook_1991_Vet.Hum.Toxicol_33_1 |
| ESTHER : Cook_1991_Vet.Hum.Toxicol_33_1 |
| PubMedSearch : Cook_1991_Vet.Hum.Toxicol_33_1 |
| PubMedID: 2017858 |
| Title : Evaluation of the efficacy of two carbamates, physostigmine and pyridostigmine, when used in conjunction for protection against organophosphate exposure - Solana_1990_Fundam.Appl.Toxicol_15_814 |
| Author(s) : Solana RP , Gennings C , Carter WH, Jr. , Anderson D , Lennox WJ , Carchman RA , Harris LW |
| Ref : Fundamental & Applied Toxicology , 15 :814 , 1990 |
| Abstract : Solana_1990_Fundam.Appl.Toxicol_15_814 |
| ESTHER : Solana_1990_Fundam.Appl.Toxicol_15_814 |
| PubMedSearch : Solana_1990_Fundam.Appl.Toxicol_15_814 |
| PubMedID: 2086320 |
| Title : Interactions of pyridostigmine with cardiopulmonary systems and their relationships to plasma cholinesterase activity - Caldwell_1989_Fundam.Appl.Toxicol_12_432 |
| Author(s) : Caldwell RW , Lowensohn HS , Chryssanthis MA , Nash CB |
| Ref : Fundamental & Applied Toxicology , 12 :432 , 1989 |
| Abstract : Caldwell_1989_Fundam.Appl.Toxicol_12_432 |
| ESTHER : Caldwell_1989_Fundam.Appl.Toxicol_12_432 |
| PubMedSearch : Caldwell_1989_Fundam.Appl.Toxicol_12_432 |
| PubMedID: 2731658 |
| Title : Pyridostigmine-induced decrement in skeletal muscle contracture is not augmented by soman - Anderson_1988_Neurotoxicol_9_89 |
| Author(s) : Anderson RJ , Chamberlain WL |
| Ref : Neurotoxicology , 9 :89 , 1988 |
| Abstract : Anderson_1988_Neurotoxicol_9_89 |
| ESTHER : Anderson_1988_Neurotoxicol_9_89 |
| PubMedSearch : Anderson_1988_Neurotoxicol_9_89 |
| PubMedID: 3393305 |
| Title : Comparison of effects of anatoxin-a(s) and paraoxon, physostigmine and pyridostigmine on mouse brain cholinesterase activity - Cook_1988_Toxicon_26_750 |
| Author(s) : Cook WO , Beasley VR , Dahlem AM , Dellinger JA , Harlin KS , Carmichael WW |
| Ref : Toxicon , 26 :750 , 1988 |
| Abstract : Cook_1988_Toxicon_26_750 |
| ESTHER : Cook_1988_Toxicon_26_750 |
| PubMedSearch : Cook_1988_Toxicon_26_750 |
| PubMedID: 3188065 |
| Title : Oxime-induced decarbamylation and atropine\/oxime therapy of guinea pigs intoxicated with pyridostigmine - Harris_1987_Life.Sci_40_577 |
| Author(s) : Harris LW , Talbot BG , Anderson DR , Lennox WJ , Green MD |
| Ref : Life Sciences , 40 :577 , 1987 |
| Abstract : Harris_1987_Life.Sci_40_577 |
| ESTHER : Harris_1987_Life.Sci_40_577 |
| PubMedSearch : Harris_1987_Life.Sci_40_577 |
| PubMedID: 3807651 |
| Title : Activation and Inhibition of the Nicotinic Receptor: Actions of Physostigmine, Pyridostigmine and Meproadifen - |
| Author(s) : Albuquerque EX , Allen CN , Aracava Y , Akaike A , Shaw KP , Rickett DL |
| Ref : Advances in Behavioral Biology , 30 :677 , 1986 |
| PubMedID: |
| Title : Metabolites of neostigmine and pyridostigmine do not contribute to antagonism of neuromuscular blockade in the dog - Hennis_1984_Anesthesiology_61_534 |
| Author(s) : Hennis PJ , Cronnelly R , Sharma M , Fisher DM , Miller RD |
| Ref : Anesthesiology , 61 :534 , 1984 |
| Abstract : Hennis_1984_Anesthesiology_61_534 |
| ESTHER : Hennis_1984_Anesthesiology_61_534 |
| PubMedSearch : Hennis_1984_Anesthesiology_61_534 |
| PubMedID: 6149707 |
| Title : The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. II. Patch clamp studies - Akaike_1984_Mol.Pharmacol_25_102 |
| Author(s) : Akaike A , Ikeda SR , Brookes N , Pascuzzo GJ , Rickett DL , Albuquerque EX |
| Ref : Molecular Pharmacology , 25 :102 , 1984 |
| Abstract : Akaike_1984_Mol.Pharmacol_25_102 |
| ESTHER : Akaike_1984_Mol.Pharmacol_25_102 |
| PubMedSearch : Akaike_1984_Mol.Pharmacol_25_102 |
| PubMedID: 6323946 |
| Title : The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. I. Agonist, desensitizing, and binding properties - Pascuzzo_1984_Mol.Pharmacol_25_92 |
| Author(s) : Pascuzzo GJ , Akaike A , Maleque MA , Shaw KP , Aronstam RS , Rickett DL , Albuquerque EX |
| Ref : Molecular Pharmacology , 25 :92 , 1984 |
| Abstract : Pascuzzo_1984_Mol.Pharmacol_25_92 |
| ESTHER : Pascuzzo_1984_Mol.Pharmacol_25_92 |
| PubMedSearch : Pascuzzo_1984_Mol.Pharmacol_25_92 |
| PubMedID: 6323955 |
| Title : Clinical pharmacology of pyridostigmine and neostigmine in patients with myasthenia gravis - Aquilonius_1983_J.Neurol.Neurosurg.Psychiatry_46_929 |
| Author(s) : Aquilonius SM , Eckernas SA , Hartvig P , Lindstrom B , Osterman PO , Stalberg E |
| Ref : Journal of Neurology Neurosurg Psychiatry , 46 :929 , 1983 |
| Abstract : Aquilonius_1983_J.Neurol.Neurosurg.Psychiatry_46_929 |
| ESTHER : Aquilonius_1983_J.Neurol.Neurosurg.Psychiatry_46_929 |
| PubMedSearch : Aquilonius_1983_J.Neurol.Neurosurg.Psychiatry_46_929 |
| PubMedID: 6644317 |
| Title : Pyridostigmine in human breast milk - |
| Author(s) : Hardell LI , Lindstrom B , Lonnerholm G , Osterman PO |
| Ref : British Journal of Clinical Pharmacology , 14 :565 , 1982 |
| PubMedID: 7138742 |
| Title : Pharmacokinetics of Neostigmine and Pyridostigmine in Man and Its Correlation to Clinical Effects in Myasthenia Gravis - |
| Author(s) : Eckernas SA , Aquilonius SM , Hartvig P , Lindstrom B , Osterman PO , Stalberg E |
| Ref : Advances in Behavioral Biology , 25 :879 , 1981 |
| PubMedID: |
| Title : Pharmacokinetics and oral bioavailability of pyridostigmine in man - Aquilonius_1980_Eur.J.Clin.Pharmacol_18_423 |
| Author(s) : Aquilonius SM , Eckernas SA , Hartvig P , Lindstrom B , Osterman PO |
| Ref : European Journal of Clinical Pharmacology , 18 :423 , 1980 |
| Abstract : Aquilonius_1980_Eur.J.Clin.Pharmacol_18_423 |
| ESTHER : Aquilonius_1980_Eur.J.Clin.Pharmacol_18_423 |
| PubMedSearch : Aquilonius_1980_Eur.J.Clin.Pharmacol_18_423 |
| PubMedID: 7439266 |
| Title : Synthesis and enzymatic evaluation of pyridostigmine analogs used to probe the active sites of acetylcholinesterase and butyrylcholinesterase - |
| Author(s) : Millner OE, Jr. , Stanley JW , Purcell WP |
| Ref : Journal of Medicinal Chemistry , 17 :13 , 1974 |
| PubMedID: 4808464 |
| Title : Placental transfer of pyridostigmine in the rat - |
| Author(s) : Roberts JB , Thomas BH , Wilson A |
| Ref : British Journal of Pharmacology , 38 :202 , 1970 |
| PubMedID: 5413286 |