Prothiofos
General
Type : Insecticide,Organophosphate,Sulfur Compound
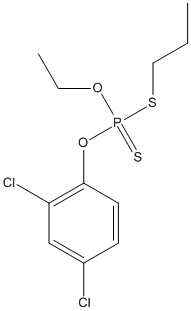
Chemical_Nomenclature : O-(2,4-Dichlorophenyl) O-ethyl S-propylphosphorodithioate
Canonical SMILES : CCCSP(=S)(OCC)OC1=C(C=C(C=C1)Cl)Cl
InChI : InChI=1S\/C11H15Cl2O2PS2\/c1-3-7-18-16(17,14-4-2)15-11-6-5-9(12)8-10(11)13\/h5-6,8H,3-4,7H2,1-2H3
InChIKey : FITIWKDOCAUBQD-UHFFFAOYSA-N
Other name(s) : Tokuthion,Prothiophos,Bideron,Dichlorpropaphos,Toyodan
MW : 345.24
Formula : C11H15Cl2O2PS2
CAS_number : 34643-46-4
PubChem : 36870
UniChem : FITIWKDOCAUBQD-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (5)
| Title : Poisoning with the S-Alkyl organophosphorus insecticides profenofos and prothiofos - Eddleston_2009_QJM_102_785 |
| Author(s) : Eddleston M , Worek F , Eyer P , Thiermann H , von Meyer L , Jeganathan K , Sheriff MH , Dawson AH , Buckley NA |
| Ref : Qjm , 102 :785 , 2009 |
| Abstract : Eddleston_2009_QJM_102_785 |
| ESTHER : Eddleston_2009_QJM_102_785 |
| PubMedSearch : Eddleston_2009_QJM_102_785 |
| PubMedID: 19737786 |
| Title : Mutations of acetylcholinesterase1 contribute to prothiofos-resistance in Plutella xylostella (L.) - Lee_2007_Biochem.Biophys.Res.Commun_353_591 |
| Author(s) : Lee DW , Choi JY , Kim WT , Je YH , Song JT , Chung BK , Boo KS , Koh YH |
| Ref : Biochemical & Biophysical Research Communications , 353 :591 , 2007 |
| Abstract : Lee_2007_Biochem.Biophys.Res.Commun_353_591 |
| ESTHER : Lee_2007_Biochem.Biophys.Res.Commun_353_591 |
| PubMedSearch : Lee_2007_Biochem.Biophys.Res.Commun_353_591 |
| PubMedID: 17196934 |
| Gene_locus related to this paper: pluxy-ACHE , pluxy-ACHE1 |
| Title : A novel function of housefly glutathione S-transferase 6B-Its effect on the retention and increase of insecticidal activity of the insecticide prothiofos - Sue_2006_J.Pestic.Sci_31_139 |
| Author(s) : Sue M , Mikawa T , Ueda T , Nomoto Y , Miyamoto T |
| Ref : Journal of Pesticide Science , 31 :139 , 2006 |
| Abstract : Sue_2006_J.Pestic.Sci_31_139 |
| ESTHER : Sue_2006_J.Pestic.Sci_31_139 |
| PubMedSearch : Sue_2006_J.Pestic.Sci_31_139 |
| PubMedID: |
| Title : Oxidative Glutathione Conjugation and Its Novel Role in Activation of the Organophosphorus Insecticide Prothiofos - Miyamoto_2005_J.Pestic.Sci_30_31 |
| Author(s) : Miyamoto T , Mikawa T |
| Ref : Journal of Pesticide Science , 30 :31 , 2005 |
| Abstract : Miyamoto_2005_J.Pestic.Sci_30_31 |
| ESTHER : Miyamoto_2005_J.Pestic.Sci_30_31 |
| PubMedSearch : Miyamoto_2005_J.Pestic.Sci_30_31 |
| PubMedID: |
| Title : Prothiofos metabolites in human poisoning - Sakata_1999_J.Toxicol.Clin.Toxicol_37_327 |
| Author(s) : Sakata M , Gotoh M , Ubukata K , Hayashi H , Kotaki M , Omote T |
| Ref : J Toxicol Clinical Toxicology , 37 :327 , 1999 |
| Abstract : Sakata_1999_J.Toxicol.Clin.Toxicol_37_327 |
| ESTHER : Sakata_1999_J.Toxicol.Clin.Toxicol_37_327 |
| PubMedSearch : Sakata_1999_J.Toxicol.Clin.Toxicol_37_327 |
| PubMedID: 10384797 |