Propaphos
General
Type : Organophosphate,Insecticide,Sulfur Compound
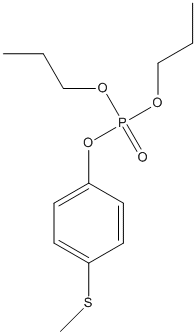
Chemical_Nomenclature : (4-methylsulfanylphenyl) dipropyl phosphate
Canonical SMILES : CCCOP(=O)(OCCC)OC1=CC=C(C=C1)SC
InChI : InChI=1S\/C13H21O4PS\/c1-4-10-15-18(14,16-11-5-2)17-12-6-8-13(19-3)9-7-12\/h6-9H,4-5,10-11H2,1-3H3
InChIKey : PWYIUEFFPNVCMW-UHFFFAOYSA-N
Other name(s) : 4-methylthiophenyl dipropylphosphate,Propafos,Kayphosnac,Kayaphos,4-Methylthiophenyl dipropyl phosphate
MW : 304.34
Formula : C13H21O4PS
CAS_number : 7292-16-2
PubChem : 23717
UniChem : PWYIUEFFPNVCMW-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (4)
| Title : Effects of fenthion, isoxathion, dichlorvos and propaphos on the serum cholinesterase isoenzyme patterns of dogs - Sakaguchi_1997_Vet.Hum.Toxicol_39_1 |
| Author(s) : Sakaguchi K , Nagayama M , Masaoka T , Nishimura A , Kageyama K , Shirai M , Akahori F |
| Ref : Vet Hum Toxicol , 39 :1 , 1997 |
| Abstract : Sakaguchi_1997_Vet.Hum.Toxicol_39_1 |
| ESTHER : Sakaguchi_1997_Vet.Hum.Toxicol_39_1 |
| PubMedSearch : Sakaguchi_1997_Vet.Hum.Toxicol_39_1 |
| PubMedID: 9004458 |
| Title : Effects of selected organophosphate insecticides on serum cholinesterase isoenzyme patterns in the rat - Nagayama_1996_Vet.Hum.Toxicol_38_196 |
| Author(s) : Nagayama M , Akahori F , Chiwata H , Shirai M , Motoya M , Masaoka T , Sakaguchi K |
| Ref : Vet Hum Toxicol , 38 :196 , 1996 |
| Abstract : Nagayama_1996_Vet.Hum.Toxicol_38_196 |
| ESTHER : Nagayama_1996_Vet.Hum.Toxicol_38_196 |
| PubMedSearch : Nagayama_1996_Vet.Hum.Toxicol_38_196 |
| PubMedID: 8727219 |
| Title : Stereoselectivity in metabolic sulfoxidation of propaphos and biological activity of chiral propaphos sulfoxide - Miyazaki_1989_Pestic.Biochem.Physiol_33_11 |
| Author(s) : Miyazaki A , Nakamura T , Marumo S |
| Ref : Pesticide Biochemistry and Physiology , 33 :11 , 1989 |
| Abstract : Miyazaki_1989_Pestic.Biochem.Physiol_33_11 |
| ESTHER : Miyazaki_1989_Pestic.Biochem.Physiol_33_11 |
| PubMedSearch : Miyazaki_1989_Pestic.Biochem.Physiol_33_11 |
| PubMedID: |
| Title : An alteration in sensitivity to cholinergic agents on guinea-pig ilea and atria after repeated administration of an organophosphate and an antagonism by a carbamate - Yamada_1979_Arch.Int.Pharmacodyn.Ther_241_32 |
| Author(s) : Yamada S , Okudaira H , Hayashi E |
| Ref : Archives Internationales de Pharmacodynamie et de Therapie , 241 :32 , 1979 |
| Abstract : Yamada_1979_Arch.Int.Pharmacodyn.Ther_241_32 |
| ESTHER : Yamada_1979_Arch.Int.Pharmacodyn.Ther_241_32 |
| PubMedSearch : Yamada_1979_Arch.Int.Pharmacodyn.Ther_241_32 |
| PubMedID: 526070 |