Pro-boropro
General
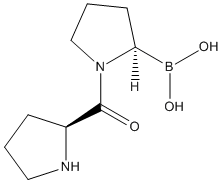
Type : Peptide,Pyrrolidine,Boron compound
Chemical_Nomenclature : [(2R)-1-[(2S)-pyrrolidine-2-carbonyl]pyrrolidin-2-yl]boronic acid
Canonical SMILES : B(C1CCCN1C(=O)C2CCCN2)(O)O
InChI : InChI=1S\/C9H17BN2O3\/c13-9(7-3-1-5-11-7)12-6-2-4-8(12)10(14)15\/h7-8,11,14-15H,1-6H2\/t7-,8-\/m0\/s1
InChIKey : XSBZZZGVAIXJLD-YUMQZZPRSA-N
Other name(s) : L-Pro-boro-L-Pro,boroPro,CHEMBL63895,CHEBI:41285,(2R)-N-[(2R)-2-(DIHYDROXYBORYL)-1-L-PROLYLPYRROLIDIN-2-YL]-N-[(5R)-5-(DIHYDROXYBORYL)-1-L-PROLYLPYRROLIDIN-2-YL]-L-PROLINAMIDE,SCHEMBL8234730,CTK0E8496
MW : 212.05
Formula : C9H17BN2O3
CAS_number :
PubChem : 10198228
UniChem : XSBZZZGVAIXJLD-YUMQZZPRSA-N
IUPHAR :
Wikipedia :
Target
Families : Pro-boropro ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 2AJD Porcine dipeptidyl peptidase IV (CD26) in complex with L-Pro-boro-L-Pro (boroPro)
Protein : pig-dpp4
References (6)
| Title : Rigidity and flexibility of dipeptidyl peptidase IV: crystal structures of and docking experiments with DPIV - Engel_2006_J.Mol.Biol_355_768 |
| Author(s) : Engel M , Hoffmann T , Manhart S , Heiser U , Chambre S , Huber R , Demuth HU , Bode W |
| Ref : Journal of Molecular Biology , 355 :768 , 2006 |
| Abstract : Engel_2006_J.Mol.Biol_355_768 |
| ESTHER : Engel_2006_J.Mol.Biol_355_768 |
| PubMedSearch : Engel_2006_J.Mol.Biol_355_768 |
| PubMedID: 16330047 |
| Gene_locus related to this paper: pig-dpp4 |
| Title : Inhibition of CD26 enzyme activity with pro-boropro stimulates rat granulocyte\/macrophage colony formation and thymocyte proliferation in vitro - Bristol_1995_Blood_85_3602 |
| Author(s) : Bristol LA , Bachovchin W , Takacs L |
| Ref : Blood , 85 :3602 , 1995 |
| Abstract : Bristol_1995_Blood_85_3602 |
| ESTHER : Bristol_1995_Blood_85_3602 |
| PubMedSearch : Bristol_1995_Blood_85_3602 |
| PubMedID: 7780144 |
| Title : Solution structures of active and inactive forms of the DP IV (CD26) inhibitor Pro-boroPro determined by NMR spectroscopy - Sudmeier_1994_Biochemistry_33_12427 |
| Author(s) : Sudmeier JL , Gunther UL , Gutheil WG , Coutts SJ , Snow RJ , Barton RW , Bachovchin WW |
| Ref : Biochemistry , 33 :12427 , 1994 |
| Abstract : Sudmeier_1994_Biochemistry_33_12427 |
| ESTHER : Sudmeier_1994_Biochemistry_33_12427 |
| PubMedSearch : Sudmeier_1994_Biochemistry_33_12427 |
| PubMedID: 7918465 |
| Title : Dipeptidyl peptidase IV (DP IV) activity in serum and on lymphocytes of MRL\/Mp-lpr\/lpr mice correlates with disease onset - Kubota_1994_Clin.Exp.Immunol_96_292 |
| Author(s) : Kubota T , Iizuka H , Bachovchin WW , Stollar BD |
| Ref : Clinical & Experimental Immunology , 96 :292 , 1994 |
| Abstract : Kubota_1994_Clin.Exp.Immunol_96_292 |
| ESTHER : Kubota_1994_Clin.Exp.Immunol_96_292 |
| PubMedSearch : Kubota_1994_Clin.Exp.Immunol_96_292 |
| PubMedID: 7910536 |
| Title : Purified and cell-bound CD26: enzymatic inhibition, antibody binding profile, and expression on T cells in relation to other surface markers - Scharpe_1994_Verh.K.Acad.Geneeskd.Belg_56_537 |
| Author(s) : Scharpe S , De Meester I |
| Ref : Verh K Acad Geneeskd Belg , 56 :537 , 1994 |
| Abstract : Scharpe_1994_Verh.K.Acad.Geneeskd.Belg_56_537 |
| ESTHER : Scharpe_1994_Verh.K.Acad.Geneeskd.Belg_56_537 |
| PubMedSearch : Scharpe_1994_Verh.K.Acad.Geneeskd.Belg_56_537 |
| PubMedID: 7892748 |
| Title : Separation of L-Pro-DL-boroPro into its component diastereomers and kinetic analysis of their inhibition of dipeptidyl peptidase IV. A new method for the analysis of slow, tight-binding inhibition - Gutheil_1993_Biochemistry_32_8723 |
| Author(s) : Gutheil WG , Bachovchin WW |
| Ref : Biochemistry , 32 :8723 , 1993 |
| Abstract : Gutheil_1993_Biochemistry_32_8723 |
| ESTHER : Gutheil_1993_Biochemistry_32_8723 |
| PubMedSearch : Gutheil_1993_Biochemistry_32_8723 |
| PubMedID: 8103356 |