Phenserine
Derivative of Physostigmine (Eserine) || Phenserine is under development by Axonyx, a US biopharmaceutical company that focuses on treatments for dementia. Phenserine is a next generation acetylcholinesterase (AChE) inhibitor indicated for the treatment of AD. Unlike currently marketed AChE inhibitors, it has a dual mechanism of action that also includes anti-amyloid activity, which may confer disease-modifying effects in patients with AD. If this is substantiated in an ongoing clinical trial then phenserine may open the door to an entirely new type of treatment for AD. Axonyx announced on 20 September 2005 that phenserine was ineffective in two curtailed phase 3 trials.
General
Type : Derivative of physostigmine-eserine,Carbamate,Drug,Indole
Chemical_Nomenclature : Pyrrolo(2,3-b)indol-5-ol, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, phenylcarbamate (ester), (3aS-cis)-
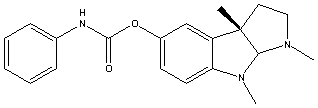
Canonical SMILES : CC12CCN(C1N(C3=C2C=C(C=C3)OC(=O)NC4=CC=CC=C4)C)C
InChI : InChI=1S\/C20H23N3O2\/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14\/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)\/t18-,20+\/m1\/s1
InChIKey : PBHFNBQPZCRWQP-QUCCMNQESA-N
Other name(s) : (-)-Phenserine,(-)-Eseroline phenylcarbamate,N-phenylcarbamoyl eseroline
MW : 337.42
Formula : C20H23N3O2
CAS_number : 101246-66-6
PubChem : 192706
UniChem : PBHFNBQPZCRWQP-QUCCMNQESA-N
IUPHAR :
Wikipedia :
Target
References (19)
| Title : Drug Repurposing in the Treatment of Traumatic Brain Injury - Ghiam_2021_Front.Neurosci_15_635483 |
| Author(s) : Ghiam MK , Patel SD , Hoffer A , Selman WR , Hoffer BJ , Hoffer ME |
| Ref : Front Neurosci , 15 :635483 , 2021 |
| Abstract : Ghiam_2021_Front.Neurosci_15_635483 |
| ESTHER : Ghiam_2021_Front.Neurosci_15_635483 |
| PubMedSearch : Ghiam_2021_Front.Neurosci_15_635483 |
| PubMedID: 33833663 |
| Title : (-)-Phenserine Ameliorates Contusion Volume, Neuroinflammation, and Behavioral Impairments Induced by Traumatic Brain Injury in Mice - Hsueh_2019_Cell.Transplant__963689719854693 |
| Author(s) : Hsueh SC , Lecca D , Greig NH , Wang JY , Selman W , Hoffer BJ , Miller JP , Chiang YH |
| Ref : Cell Transplantation , :963689719854693 , 2019 |
| Abstract : Hsueh_2019_Cell.Transplant__963689719854693 |
| ESTHER : Hsueh_2019_Cell.Transplant__963689719854693 |
| PubMedSearch : Hsueh_2019_Cell.Transplant__963689719854693 |
| PubMedID: 31177840 |
| Title : (-)-Phenserine inhibits neuronal apoptosis following ischemia\/reperfusion injury - Chang_2017_Brain.Res_1677_118 |
| Author(s) : Chang CF , Lai JH , Wu JC , Greig NH , Becker RE , Luo Y , Chen YH , Kang SJ , Chiang YH , Chen KY |
| Ref : Brain Research , 1677 :118 , 2017 |
| Abstract : Chang_2017_Brain.Res_1677_118 |
| ESTHER : Chang_2017_Brain.Res_1677_118 |
| PubMedSearch : Chang_2017_Brain.Res_1677_118 |
| PubMedID: 28963051 |
| Title : Differential effects of two hexahydropyrroloindole carbamate-based anticholinesterase drugs on the amyloid beta protein pathway involved in Alzheimer's disease - Lahiri_2007_Neuromolecular.Med_9_157 |
| Author(s) : Lahiri DK , Alley GM , Tweedie D , Chen D , Greig NH |
| Ref : Neuromolecular Med , 9 :157 , 2007 |
| Abstract : Lahiri_2007_Neuromolecular.Med_9_157 |
| ESTHER : Lahiri_2007_Neuromolecular.Med_9_157 |
| PubMedSearch : Lahiri_2007_Neuromolecular.Med_9_157 |
| PubMedID: 17627035 |
| Title : Phenserine Axonyx - Thatte_2005_Curr.Opin.Investig.Drugs_6_729 |
| Author(s) : Thatte U |
| Ref : Curr Opin Investig Drugs , 6 :729 , 2005 |
| Abstract : Thatte_2005_Curr.Opin.Investig.Drugs_6_729 |
| ESTHER : Thatte_2005_Curr.Opin.Investig.Drugs_6_729 |
| PubMedSearch : Thatte_2005_Curr.Opin.Investig.Drugs_6_729 |
| PubMedID: 16044670 |
| Title : An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer's disease - Greig_2005_Curr.Alzheimer.Res_2_281 |
| Author(s) : Greig NH , Sambamurti K , Yu QS , Brossi A , Bruinsma GB , Lahiri DK |
| Ref : Curr Alzheimer Res , 2 :281 , 2005 |
| Abstract : Greig_2005_Curr.Alzheimer.Res_2_281 |
| ESTHER : Greig_2005_Curr.Alzheimer.Res_2_281 |
| PubMedSearch : Greig_2005_Curr.Alzheimer.Res_2_281 |
| PubMedID: 15974893 |
| Title : Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects - Greig_2005_Curr.Alzheimer.Res_2_483 |
| Author(s) : Greig NH , Ruckle J , Comer P , Brownell L , Holloway HW , Flanagan DR, Jr. , Canfield CJ , Burford RG |
| Ref : Curr Alzheimer Res , 2 :483 , 2005 |
| Abstract : Greig_2005_Curr.Alzheimer.Res_2_483 |
| ESTHER : Greig_2005_Curr.Alzheimer.Res_2_483 |
| PubMedSearch : Greig_2005_Curr.Alzheimer.Res_2_483 |
| PubMedID: 16248851 |
| Title : The cholinesterase inhibitor, phenserine, improves Morris water maze performance of scopolamine-treated rats - Janas_2005_Life.Sci_76_1073 |
| Author(s) : Janas AM , Cunningham SC , Duffy KB , Devan BD , Greig NH , Holloway HW , Yu QS , Markowska AL , Ingram DK , Spangler EL |
| Ref : Life Sciences , 76 :1073 , 2005 |
| Abstract : Janas_2005_Life.Sci_76_1073 |
| ESTHER : Janas_2005_Life.Sci_76_1073 |
| PubMedSearch : Janas_2005_Life.Sci_76_1073 |
| PubMedID: 15620572 |
| Title : Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development - Shaw_2001_Proc.Natl.Acad.Sci.U.S.A_98_7605 |
| Author(s) : Shaw KT , Utsuki T , Rogers J , Yu QS , Sambamurti K , Brossi A , Ge YW , Lahiri DK , Greig NH |
| Ref : Proc Natl Acad Sci U S A , 98 :7605 , 2001 |
| Abstract : Shaw_2001_Proc.Natl.Acad.Sci.U.S.A_98_7605 |
| ESTHER : Shaw_2001_Proc.Natl.Acad.Sci.U.S.A_98_7605 |
| PubMedSearch : Shaw_2001_Proc.Natl.Acad.Sci.U.S.A_98_7605 |
| PubMedID: 11404470 |
| Title : Methyl analogues of the experimental Alzheimer drug phenserine: synthesis and structure\/activity relationships for acetyl- and butyrylcholinesterase inhibitory action - Yu_2001_J.Med.Chem_44_4062 |
| Author(s) : Yu Q , Holloway HW , Flippen-Anderson JL , Hoffman B , Brossi A , Greig NH |
| Ref : Journal of Medicinal Chemistry , 44 :4062 , 2001 |
| Abstract : Yu_2001_J.Med.Chem_44_4062 |
| ESTHER : Yu_2001_J.Med.Chem_44_4062 |
| PubMedSearch : Yu_2001_J.Med.Chem_44_4062 |
| PubMedID: 11708910 |
| Title : The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics - Greig_2000_Acta.Neurol.Scand.Suppl_176_74 |
| Author(s) : Greig NH , De Micheli E , Holloway HW , Yu QS , Utsuki T , Perry TA , Brossi A , Ingram DK , Deutsch J , Lahiri DK , Soncrant TT |
| Ref : Acta Neurologica Scandinavica Supplementum , 176 :74 , 2000 |
| Abstract : Greig_2000_Acta.Neurol.Scand.Suppl_176_74 |
| ESTHER : Greig_2000_Acta.Neurol.Scand.Suppl_176_74 |
| PubMedSearch : Greig_2000_Acta.Neurol.Scand.Suppl_176_74 |
| PubMedID: 11261809 |
| Title : Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer's disease - Yu_1999_J.Med.Chem_42_1855 |
| Author(s) : Yu Q , Holloway HW , Utsuki T , Brossi A , Greig NH |
| Ref : Journal of Medicinal Chemistry , 42 :1855 , 1999 |
| Abstract : Yu_1999_J.Med.Chem_42_1855 |
| ESTHER : Yu_1999_J.Med.Chem_42_1855 |
| PubMedSearch : Yu_1999_J.Med.Chem_42_1855 |
| PubMedID: 10346939 |
| Title : Phenserine, a novel acetylcholinesterase inhibitor, attenuates impaired learning of rats in a 14-unit T-maze induced by blockade of the N- methyl-D-aspartate receptor - Patel_1998_Neuroreport_9_171 |
| Author(s) : Patel N , Spangler EL , Greig NH , Yu QS , Ingram DK , Meyer RC |
| Ref : Neuroreport , 9 :171 , 1998 |
| Abstract : Patel_1998_Neuroreport_9_171 |
| ESTHER : Patel_1998_Neuroreport_9_171 |
| PubMedSearch : Patel_1998_Neuroreport_9_171 |
| PubMedID: 9592071 |
| Title : Kinetics of human erythrocyte acetylcholinesterase inhibition by a novel derivative of physostigmine: phenserine - al-Jafari_1998_Biochem.Biophys.Res.Commun_248_180 |
| Author(s) : Al-Jafari AA , Kamal MA , Greig NH , Alhomida AS , Perry ER |
| Ref : Biochemical & Biophysical Research Communications , 248 :180 , 1998 |
| Abstract : al-Jafari_1998_Biochem.Biophys.Res.Commun_248_180 |
| ESTHER : al-Jafari_1998_Biochem.Biophys.Res.Commun_248_180 |
| PubMedSearch : al-Jafari_1998_Biochem.Biophys.Res.Commun_248_180 |
| PubMedID: 9675107 |
| Title : Syntheses and anticholinesterase activities of (3aS)-N1, N8- bisnorphenserine, (3aS)-N1,N8-bisnorphysostigmine, their antipodal isomers, and other potential metabolites of phenserine - Yu_1998_J.Med.Chem_41_2371 |
| Author(s) : Yu Q , Greig NH , Holloway HW , Brossi A |
| Ref : Journal of Medicinal Chemistry , 41 :2371 , 1998 |
| Abstract : Yu_1998_J.Med.Chem_41_2371 |
| ESTHER : Yu_1998_J.Med.Chem_41_2371 |
| PubMedSearch : Yu_1998_J.Med.Chem_41_2371 |
| PubMedID: 9632370 |
| Title : Total syntheses and anticholinesterase activities of (3aS)-N(8)- norphysostigmine, (3aS)-N(8)-norphenserine, their antipodal isomers, and other N(8)-substituted analogues - Yu_1997_J.Med.Chem_40_2895 |
| Author(s) : Yu QS , Pei XF , Holloway HW , Greig NH , Brossi A |
| Ref : Journal of Medicinal Chemistry , 40 :2895 , 1997 |
| Abstract : Yu_1997_J.Med.Chem_40_2895 |
| ESTHER : Yu_1997_J.Med.Chem_40_2895 |
| PubMedSearch : Yu_1997_J.Med.Chem_40_2895 |
| PubMedID: 9288171 |
| Title : Maze learning in aged rats is enhanced by phenserine, a novel anticholinesterase - Ikari_1995_Neuroreport_6_481 |
| Author(s) : Ikari H , Spangler EL , Greig NH , Pei XF , Brossi A , Speer D , Patel N , Ingram DK |
| Ref : Neuroreport , 6 :481 , 1995 |
| Abstract : Ikari_1995_Neuroreport_6_481 |
| ESTHER : Ikari_1995_Neuroreport_6_481 |
| PubMedSearch : Ikari_1995_Neuroreport_6_481 |
| PubMedID: 7766848 |
| Title : Phenserine: a physostigmine derivative that is a long-acting inhibitor of cholinesterase and demonstrates a wide dose range for attenuating a scopolamine-induced learning impairment of rats in a 14-unit T-maze - Iijima_1993_Psychopharmacology_112_415 |
| Author(s) : Iijima S , Greig NH , Garofalo P , Spangler EL , Heller B , Brossi A , Ingram DK |
| Ref : Psychopharmacology , 112 :415 , 1993 |
| Abstract : Iijima_1993_Psychopharmacology_112_415 |
| ESTHER : Iijima_1993_Psychopharmacology_112_415 |
| PubMedSearch : Iijima_1993_Psychopharmacology_112_415 |
| PubMedID: 7871051 |