Palmatine
Palmatine is a berberine alkaloid and an organic heterotetracyclic compound. It has a role as a plant metabolite. || IC50 ACHE 1.7 muM Hostalkova et al.
General
Type : Natural,Alkaloid,Not A\/B H target,Isoquinoline
Chemical_Nomenclature : 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium
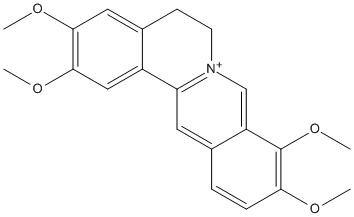
Canonical SMILES : COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC(=C(C=C4CC3)OC)OC)OC
InChI : InChI=1S\/C21H22NO4\/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4\/h5-6,9-12H,7-8H2,1-4H3\/q+1
InChIKey : QUCQEUCGKKTEBI-UHFFFAOYSA-N
Other name(s) : Berbericinine,O,O-Dimethyldemethyleneberberine,Burasaine
MW : 352.4
Formula : C21H22NO4+
CAS_number : 3486-67-7
PubChem : 19009
UniChem : QUCQEUCGKKTEBI-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (22)
| Title : Binding interaction of protoberberine alkaloids against acetylcholinesterase (AChE) using molecular dynamics simulations and QM\/MM calculations - Honorio_2021_Chem.Biol.Interact__109523 |
| Author(s) : Honorio P , Sainimnuan S , Hannongbua S , Saparpakorn P |
| Ref : Chemico-Biological Interactions , :109523 , 2021 |
| Abstract : Honorio_2021_Chem.Biol.Interact__109523 |
| ESTHER : Honorio_2021_Chem.Biol.Interact__109523 |
| PubMedSearch : Honorio_2021_Chem.Biol.Interact__109523 |
| PubMedID: 34033838 |
| Title : Palmatine antioxidant and anti-acetylcholinesterase activities: A pre-clinical assessment - Chaves_2020_Cell.Mol.Biol.(Noisy-le-grand)_66_54 |
| Author(s) : Chaves SKM , Afzal MI , Islam MT , Hameed A , Da Mata A , Da Silva Araujo L , Ali SW , Rolim HML , De Medeiros M , Costa EV , Salehi B , Martins N , Arif AM , Imran M , Sharifi-Rad J , Melo-Cavalcante AAC , Feitosa CM |
| Ref : Cellular & Molecular Biology (Noisy-le-grand) , 66 :54 , 2020 |
| Abstract : Chaves_2020_Cell.Mol.Biol.(Noisy-le-grand)_66_54 |
| ESTHER : Chaves_2020_Cell.Mol.Biol.(Noisy-le-grand)_66_54 |
| PubMedSearch : Chaves_2020_Cell.Mol.Biol.(Noisy-le-grand)_66_54 |
| PubMedID: 32583771 |
| Title : Screening of acetylcholinesterase inhibitors and characterizing of phytochemical constituents from Dichocarpum auriculatum (Franch.) W.T. Wang & P. K. Hsiao through UPLC-MS combined with an acetylcholinesterase inhibition assay in vitro - Li_2019_J.Ethnopharmacol_245_112185 |
| Author(s) : Li P , Liu S , Liu Q , Shen J , Yang R , Jiang B , He C , Xiao P |
| Ref : J Ethnopharmacol , 245 :112185 , 2019 |
| Abstract : Li_2019_J.Ethnopharmacol_245_112185 |
| ESTHER : Li_2019_J.Ethnopharmacol_245_112185 |
| PubMedSearch : Li_2019_J.Ethnopharmacol_245_112185 |
| PubMedID: 31446073 |
| Title : Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study - Balkrishna_2019_Molecules_24_ |
| Author(s) : Balkrishna A , Pokhrel S , Tomer M , Verma S , Kumar A , Nain P , Gupta A , Varshney A |
| Ref : Molecules , 24 : , 2019 |
| Abstract : Balkrishna_2019_Molecules_24_ |
| ESTHER : Balkrishna_2019_Molecules_24_ |
| PubMedSearch : Balkrishna_2019_Molecules_24_ |
| PubMedID: 31752124 |
| Title : Acetylcholinesterase inhibitory activities and bioguided fractionation of the Ocotea percoriacea extracts: HPLC-DAD-MS\/MS characterization and molecular modeling of their alkaloids in the active fraction - Cassiano_2019_Comput.Biol.Chem_83_107129 |
| Author(s) : Cassiano DSA , Reis IMA , Estrela IO , de Freitas HF , Pita S , David JM , Branco A |
| Ref : Comput Biol Chem , 83 :107129 , 2019 |
| Abstract : Cassiano_2019_Comput.Biol.Chem_83_107129 |
| ESTHER : Cassiano_2019_Comput.Biol.Chem_83_107129 |
| PubMedSearch : Cassiano_2019_Comput.Biol.Chem_83_107129 |
| PubMedID: 31606587 |
| Title : Large-scale separation of acetylcholinesterase inhibitors from Zanthoxylum nitidum by pH-zone-refining counter-current chromatography target-guided by ultrafiltration high-performance liquid chromatography with ultraviolet and mass spectrometry screening - Liu_2019_J.Sep.Sci_42_1194 |
| Author(s) : Liu M , Liu Q , Chen M , Huang X , Chen X |
| Ref : J Sep Sci , 42 :1194 , 2019 |
| Abstract : Liu_2019_J.Sep.Sci_42_1194 |
| ESTHER : Liu_2019_J.Sep.Sci_42_1194 |
| PubMedSearch : Liu_2019_J.Sep.Sci_42_1194 |
| PubMedID: 30638299 |
| Title : Isoquinoline Alkaloids from Berberis vulgaris as Potential Lead Compounds for the Treatment of Alzheimer's Disease - Hostalkova_2019_J.Nat.Prod_82_239 |
| Author(s) : Hostalkova A , Marikova J , Opletal L , Korabecny J , Hulcova D , Kunes J , Novakova L , Perez DI , Jun D , Kucera T , Andrisano V , Siatka T , Cahlikova L |
| Ref : Journal of Natural Products , 82 :239 , 2019 |
| Abstract : Hostalkova_2019_J.Nat.Prod_82_239 |
| ESTHER : Hostalkova_2019_J.Nat.Prod_82_239 |
| PubMedSearch : Hostalkova_2019_J.Nat.Prod_82_239 |
| PubMedID: 30701972 |
| Title : Protective effects of tetrahydropalmatine against ketamine-induced learning and memory injury via antioxidative, anti-inflammatory and anti-apoptotic mechanisms in mice - Zhang_2018_Mol.Med.Rep_17_6873 |
| Author(s) : Zhang Y , Sha R , Wang K , Li H , Yan B , Zhou N |
| Ref : Mol Med Rep , 17 :6873 , 2018 |
| Abstract : Zhang_2018_Mol.Med.Rep_17_6873 |
| ESTHER : Zhang_2018_Mol.Med.Rep_17_6873 |
| PubMedSearch : Zhang_2018_Mol.Med.Rep_17_6873 |
| PubMedID: 29512789 |
| Title : Online screening of acetylcholinesterase inhibitors in natural products using monolith-based immobilized capillary enzyme reactors combined with liquid chromatography-mass spectrometry - Wang_2018_J.Chromatogr.A_1563_135 |
| Author(s) : Wang L , Zhao Y , Zhang Y , Zhang T , Kool J , Somsen GW , Wang Q , Jiang Z |
| Ref : Journal of Chromatography A , 1563 :135 , 2018 |
| Abstract : Wang_2018_J.Chromatogr.A_1563_135 |
| ESTHER : Wang_2018_J.Chromatogr.A_1563_135 |
| PubMedSearch : Wang_2018_J.Chromatogr.A_1563_135 |
| PubMedID: 29866504 |
| Title : Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat - Qu_2016_Physiol.Behav_154_114 |
| Author(s) : Qu Z , Zhang J , Yang H , Huo L , Gao J , Chen H , Gao W |
| Ref : Physiol Behav , 154 :114 , 2016 |
| Abstract : Qu_2016_Physiol.Behav_154_114 |
| ESTHER : Qu_2016_Physiol.Behav_154_114 |
| PubMedSearch : Qu_2016_Physiol.Behav_154_114 |
| PubMedID: 26592138 |
| Title : Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer's Disease Target - Kaufmann_2016_Molecules_21_ |
| Author(s) : Kaufmann D , Kaur Dogra A , Tahrani A , Herrmann F , Wink M |
| Ref : Molecules , 21 : , 2016 |
| Abstract : Kaufmann_2016_Molecules_21_ |
| ESTHER : Kaufmann_2016_Molecules_21_ |
| PubMedSearch : Kaufmann_2016_Molecules_21_ |
| PubMedID: 27589716 |
| Title : An in vitro AChE inhibition assay combined with UF-HPLC-ESI-Q-TOF\/MS approach for screening and characterizing of AChE inhibitors from roots of Coptis chinensis Franch - Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| Author(s) : Zhao H , Zhou S , Zhang M , Feng J , Wang S , Wang D , Geng Y , Wang X |
| Ref : J Pharm Biomed Anal , 120 :235 , 2015 |
| Abstract : Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| ESTHER : Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| PubMedSearch : Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| PubMedID: 26760241 |
| Title : Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from chinese medicinal herbs - Mak_2014_J.Mol.Neurosci_53_511 |
| Author(s) : Mak SH , Luk WW , Cui W , Hu S , Tsim KWK , Han Y |
| Ref : Journal of Molecular Neuroscience , 53 :511 , 2014 |
| Abstract : Mak_2014_J.Mol.Neurosci_53_511 |
| ESTHER : Mak_2014_J.Mol.Neurosci_53_511 |
| PubMedSearch : Mak_2014_J.Mol.Neurosci_53_511 |
| PubMedID: 24793543 |
| Title : Berberis aetnensis and B. libanotica: a comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer's disease and antioxidant activity - Bonesi_2013_J.Pharm.Pharmacol_65_1726 |
| Author(s) : Bonesi M , Loizzo MR , Conforti F , Passalacqua NG , Saab A , Menichini F , Tundis R |
| Ref : J Pharm Pharmacol , 65 :1726 , 2013 |
| Abstract : Bonesi_2013_J.Pharm.Pharmacol_65_1726 |
| ESTHER : Bonesi_2013_J.Pharm.Pharmacol_65_1726 |
| PubMedSearch : Bonesi_2013_J.Pharm.Pharmacol_65_1726 |
| PubMedID: 24236982 |
| Title : Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: The case of chelerythrine - Brunhofer_2012_Bioorg.Med.Chem_20_6669 |
| Author(s) : Brunhofer G , Fallarero A , Karlsson D , Batista-Gonzalez A , Shinde P , Gopi Mohan C , Vuorela P |
| Ref : Bioorganic & Medicinal Chemistry , 20 :6669 , 2012 |
| Abstract : Brunhofer_2012_Bioorg.Med.Chem_20_6669 |
| ESTHER : Brunhofer_2012_Bioorg.Med.Chem_20_6669 |
| PubMedSearch : Brunhofer_2012_Bioorg.Med.Chem_20_6669 |
| PubMedID: 23062825 |
| Title : Bioactive isoquinoline alkaloids from Corydalis saxicola - Huang_2012_Planta.Med_78_65 |
| Author(s) : Huang QQ , Bi JL , Sun QY , Yang FM , Wang YH , Tang GH , Zhao FW , Wang H , Xu JJ , Kennelly EJ , Long CL , Yin GF |
| Ref : Planta Med , 78 :65 , 2012 |
| Abstract : Huang_2012_Planta.Med_78_65 |
| ESTHER : Huang_2012_Planta.Med_78_65 |
| PubMedSearch : Huang_2012_Planta.Med_78_65 |
| PubMedID: 21858757 |
| Title : Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze - Dhingra_2012_Adv.Pharmacol.Sci_2012_357368 |
| Author(s) : Dhingra D , Kumar V |
| Ref : Advances in Pharmacology Sci , 2012 :357368 , 2012 |
| Abstract : Dhingra_2012_Adv.Pharmacol.Sci_2012_357368 |
| ESTHER : Dhingra_2012_Adv.Pharmacol.Sci_2012_357368 |
| PubMedSearch : Dhingra_2012_Adv.Pharmacol.Sci_2012_357368 |
| PubMedID: 23193393 |
| Title : Acetylcholinesterase inhibitors from Corydalis yanhusuo - Xiao_2011_Nat.Prod.Res_25_1418 |
| Author(s) : Xiao HT , Peng J , Liang Y , Yang J , Bai X , Hao XY , Yang FM , Sun QY |
| Ref : Nat Prod Res , 25 :1418 , 2011 |
| Abstract : Xiao_2011_Nat.Prod.Res_25_1418 |
| ESTHER : Xiao_2011_Nat.Prod.Res_25_1418 |
| PubMedSearch : Xiao_2011_Nat.Prod.Res_25_1418 |
| PubMedID: 20234973 |
| Title : Characterization of Acetylcholinesterase Inhibitory Constituents from Annona glabra Assisted by HPLC Microfractionation - Tsai_2010_J.Nat.Prod_73_1632 |
| Author(s) : Tsai SF , Lee SS |
| Ref : Journal of Natural Products , 73 :1632 , 2010 |
| Abstract : Tsai_2010_J.Nat.Prod_73_1632 |
| ESTHER : Tsai_2010_J.Nat.Prod_73_1632 |
| PubMedSearch : Tsai_2010_J.Nat.Prod_73_1632 |
| PubMedID: 20828184 |
| Title : Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa - Kim_2004_Arch.Pharm.Res_27_1127 |
| Author(s) : Kim DK , Lee KT , Baek NI , Kim SH , Park HW , Lim JP , Shin TY , Eom DO , Yang JH , Eun JS |
| Ref : Arch Pharm Res , 27 :1127 , 2004 |
| Abstract : Kim_2004_Arch.Pharm.Res_27_1127 |
| ESTHER : Kim_2004_Arch.Pharm.Res_27_1127 |
| PubMedSearch : Kim_2004_Arch.Pharm.Res_27_1127 |
| PubMedID: 15595415 |
| Title : Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protoberberine alkaloids - Shigeta_2002_Biosci.Biotechnol.Biochem_66_2491 |
| Author(s) : Shigeta K , Ootaki K , Tatemoto H , Nakanishi T , Inada A , Muto N |
| Ref : Biosci Biotechnol Biochem , 66 :2491 , 2002 |
| Abstract : Shigeta_2002_Biosci.Biotechnol.Biochem_66_2491 |
| ESTHER : Shigeta_2002_Biosci.Biotechnol.Biochem_66_2491 |
| PubMedSearch : Shigeta_2002_Biosci.Biotechnol.Biochem_66_2491 |
| PubMedID: 12506995 |
| Title : Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores - Schmeller_1997_Phytochemistry_44_257 |
| Author(s) : Schmeller T , Latz-Bruning B , Wink M |
| Ref : Phytochemistry , 44 :257 , 1997 |
| Abstract : Schmeller_1997_Phytochemistry_44_257 |
| ESTHER : Schmeller_1997_Phytochemistry_44_257 |
| PubMedSearch : Schmeller_1997_Phytochemistry_44_257 |
| PubMedID: 9004542 |