Pa5
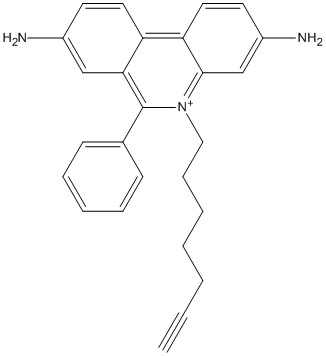
AChE PAS inhibitor (phenylphenanthridinium) equipped with an acatylene group at the end of a flexible methylene chain used as building block of 1,3-dipolar cycloaddition (click chemistry) with an active site inhibitor (tacrine) equipped with an azide group at the end of a methylene chain
General
Type : Phenanthridinium,Alkyne
Chemical_Nomenclature : 5-hept-6-ynyl-6-phenylphenanthridin-5-ium-3,8-diamine
Canonical SMILES : C#CCCCCC[N+]1=C2C=C(C=CC2=C3C=CC(=CC3=C1C4=CC=CC=C4)N)N
InChI : InChI=1S\/C26H25N3\/c1-2-3-4-5-9-16-29-25-18-21(28)13-15-23(25)22-14-12-20(27)17-24(22)26(29)19-10-7-6-8-11-19\/h1,6-8,10-15,17-18,28H,3-5,9,16,27H2\/p+1
InChIKey : WJXZOVZRCNSPKF-UHFFFAOYSA-O
Other name(s) : 3,8-Diamino-5-(6-heptynyl)-6-phenylphenanthridinium,3,8-diamino-5-oct-7'-ynyl-5-phenylphenanthridinium triflate,PZ5
MW : 380.51
Formula : C26H26N3
CAS_number :
PubChem :
UniChem : WJXZOVZRCNSPKF-UHFFFAOYSA-O
IUPHAR :
Wikipedia :
References (3)
| Title : Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation - Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| Author(s) : Bourne Y , Kolb HC , Radic Z , Sharpless KB , Taylor P , Marchot P |
| Ref : Proc Natl Acad Sci U S A , 101 :1449 , 2004 |
| Abstract : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| ESTHER : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| PubMedSearch : Bourne_2004_Proc.Natl.Acad.Sci.U.S.A_101_1449 |
| PubMedID: 14757816 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Cover picture: angew. Chem. Int. Ed. 6\/2002 - Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| Author(s) : Lewis WG , Green LG , Grynszpan F , Radic Z , Carlier PR , Taylor P , Finn MG , Sharpless KB |
| Ref : Angew Chem Int Ed Engl , 41 :875 , 2002 |
| Abstract : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| ESTHER : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| PubMedSearch : Lewis_2002_Angew.Chem.Int.Ed.Engl_41_875 |
| PubMedID: |
| Title : Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks - |
| Author(s) : Lewis WG , Green LG , Grynszpan F , Radic Z , Carlier PR , Taylor P , Finn MG , Sharpless KB |
| Ref : Angew Chem Int Ed Engl , 41 :1053 , 2002 |
| PubMedID: 12491310 |