PEG-SH-350
Polyethylene glycol (PEG) is a polyether compound with many applications from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H-(O-CH2-CH2)n-OH. The numbers that are often included in the names of PEGs indicate their average molecular weights (e.g. a PEG with n = 9 would have an average molecular weight of approximately 400 daltons, and would be labeled PEG 400.) PEG-200 n =4 190 - 210 g/mol
General
Type : Polymer,Poly-ethylene-glycol,Sulfur Compound
Chemical_Nomenclature : 2-[2-[2-[2-[2-[2-(2-sulfanylethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol
Canonical SMILES : C(COCCOCCOCCOCCOCCOCCS)O
InChI : InChI=1S\/C14H30O7S\/c15-1-2-16-3-4-17-5-6-18-7-8-19-9-10-20-11-12-21-13-14-22\/h15,22H,1-14H2
InChIKey : ACMBXVJDKVNCGH-UHFFFAOYSA-N
Other name(s) : 1-deoxy-1-thio-heptaethylene glycol,PE7,D04OUJ,AC1L9JR2,MolPort-006-168-364,ZINC5828797,DB02404
MW : 342.44
Formula : C14H30O7S
CAS_number :
PubChem : 446506
UniChem : ACMBXVJDKVNCGH-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
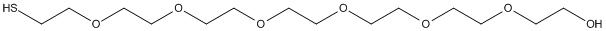
Target
Families : PEG-SH-350 ligand of proteins in family: ACHE
Stucture : 1JJB A neutral molecule in cation-binding site: Specific binding of PEG-SH to Acetylcholinesterase from Torpedo californica
Protein : torca-ACHE
References (2)
| Title : A neutral molecule in a cation-binding site: specific binding of a PEG-SH to acetylcholinesterase from Torpedo californica - Koellner_2002_J.Mol.Biol_320_721 |
| Author(s) : Koellner G , Steiner T , Millard CB , Silman I , Sussman JL |
| Ref : Journal of Molecular Biology , 320 :721 , 2002 |
| Abstract : Koellner_2002_J.Mol.Biol_320_721 |
| ESTHER : Koellner_2002_J.Mol.Biol_320_721 |
| PubMedSearch : Koellner_2002_J.Mol.Biol_320_721 |
| PubMedID: 12095250 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Active-site gorge and buried water molecules in crystal structures of acetylcholinesterase from Torpedo californica - Koellner_2000_J.Mol.Biol_296_713 |
| Author(s) : Koellner G , Kryger G , Millard CB , Silman I , Sussman JL , Steiner T |
| Ref : Journal of Molecular Biology , 296 :713 , 2000 |
| Abstract : Koellner_2000_J.Mol.Biol_296_713 |
| ESTHER : Koellner_2000_J.Mol.Biol_296_713 |
| PubMedSearch : Koellner_2000_J.Mol.Biol_296_713 |
| PubMedID: 10669619 |