P-10358
Smith et al. IC50 0.10 microM for AChE 0.08 microM for BChE selectivity 1.25 11 time less potent than heptastigmine and 2.5 more potent than Tacrine
General
Type : Carbamate,Indole,Pyridine
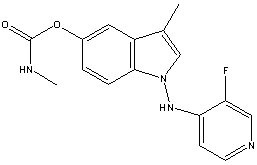
Chemical_Nomenclature : [1-[(3-fluoropyridin-4-yl)amino]-3-methylindol-5-yl] N-methylcarbamate
Canonical SMILES : CC1=CN(C2=C1C=C(C=C2)OC(=O)NC)NC3=C(C=NC=C3)F
InChI : InChI=1S\/C16H15FN4O2\/c1-10-9-21(20-14-5-6-19-8-13(14)17)15-4-3-11(7-12(10)15)23-16(22)18-2\/h3-9H,1-2H3,(H,18,22)(H,19,20)
InChIKey : GUHMRCCRDRBMHO-UHFFFAOYSA-N
Other name(s) : 1-((3-Fluoro-4-pyridinyl)amino)-3-methyl-1H-indol-5-ol methylcarbamate (ester),1H-Indol-5-ol, 1-((3-fluoro-4-pyridinyl)amino)-3-methyl-, methylcarbamate (ester)
MW : 314.314
Formula : C16H15FN4O2
CAS_number : 188240-59-7
PubChem : 3075612
UniChem : GUHMRCCRDRBMHO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Central and peripheral activity of cholinesterase inhibitors as revealed by yawning and fasciculation in rats - Ogura_2001_Eur.J.Pharmacol_415_157 |
| Author(s) : Ogura H , Kosasa T , Kuriya Y , Yamanishi Y |
| Ref : European Journal of Pharmacology , 415 :157 , 2001 |
| Abstract : Ogura_2001_Eur.J.Pharmacol_415_157 |
| ESTHER : Ogura_2001_Eur.J.Pharmacol_415_157 |
| PubMedSearch : Ogura_2001_Eur.J.Pharmacol_415_157 |
| PubMedID: 11274994 |
| Title : Pharmacological activity and safety profile of P10358, a novel, orally active acetylcholinesterase inhibitor for Alzheimer's disease - Smith_1997_J.Pharmacol.Exp.Ther_280_710 |
| Author(s) : Smith CP , Bores GM , Petko W , Li M , Selk DE , Rush DK , Camacho F , Winslow JT , Fishkin R , Cunningham DM , Brooks KM , Roehr J , Hartman HB , Davis L , Vargas HM |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 280 :710 , 1997 |
| Abstract : Smith_1997_J.Pharmacol.Exp.Ther_280_710 |
| ESTHER : Smith_1997_J.Pharmacol.Exp.Ther_280_710 |
| PubMedSearch : Smith_1997_J.Pharmacol.Exp.Ther_280_710 |
| PubMedID: 9023283 |