Oleanolic-acid
Oleanolic acid is a natural pentacyclic triterpene found in Panax ginseng, Rhabdodendron amazonicum, and in many plants as the free acid or the aglycone for many saponins.It can rearrange to the isomer, ursolic acid
General
Type : Not A\/B H target,Natural,Terpenoid
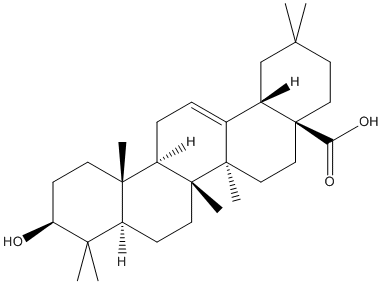
Chemical_Nomenclature : (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid
Canonical SMILES : CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1)C)C(=O)O)C
InChI : InChI=1S\/C30H48O3\/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7\/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)\/t20-,21-,22+,23-,27-,28+,29+,30-\/m0\/s1
InChIKey : MIJYXULNPSFWEK-GTOFXWBISA-N
Other name(s) : Oleanolic acid,Oleanic acid,Caryophyllin,Astrantiagenin C
MW : 456.7
Formula : C30H48O3
CAS_number : 508-02-1
PubChem : 10494
UniChem : MIJYXULNPSFWEK-GTOFXWBISA-N
IUPHAR :
Wikipedia :
Target
References (13)
| Title : Ursolic acid and its isomer oleanolic acid are responsible for the anti-dementia effects of Ocimum sanctum in olfactory bulbectomized mice - Nguyen_2022_J.Nat.Med__ |
| Author(s) : Nguyen HT , Le XT , Van Nguyen T , Phung HN , Pham HTN , Nguyen KM , Matsumoto K |
| Ref : J Nat Med , : , 2022 |
| Abstract : Nguyen_2022_J.Nat.Med__ |
| ESTHER : Nguyen_2022_J.Nat.Med__ |
| PubMedSearch : Nguyen_2022_J.Nat.Med__ |
| PubMedID: 35218459 |
| Title : Discovery of triterpenoids as potent dual inhibitors of pancreatic lipase and human carboxylesterase 1 - Zhang_2022_J.Enzyme.Inhib.Med.Chem_37_629 |
| Author(s) : Zhang J , Pan QS , Qian XK , Zhou XL , Wang YJ , He RJ , Wang LT , Li YR , Huo H , Sun CG , Sun L , Zou LW , Yang L |
| Ref : J Enzyme Inhib Med Chem , 37 :629 , 2022 |
| Abstract : Zhang_2022_J.Enzyme.Inhib.Med.Chem_37_629 |
| ESTHER : Zhang_2022_J.Enzyme.Inhib.Med.Chem_37_629 |
| PubMedSearch : Zhang_2022_J.Enzyme.Inhib.Med.Chem_37_629 |
| PubMedID: 35100926 |
| Title : Phytochemical Analysis and Evaluation of Biological Activity of Lawsonia inermis Seeds Related to Alzheimer's Disease - Balaei-Kahnamoei_2021_Evid.Based.Complement.Alternat.Med_2021_5965061 |
| Author(s) : Balaei-Kahnamoei M , Saeedi M , Rastegari A , Shams Ardekani MR , Akbarzadeh T , Khanavi M |
| Ref : Evid Based Complement Alternat Med , 2021 :5965061 , 2021 |
| Abstract : Balaei-Kahnamoei_2021_Evid.Based.Complement.Alternat.Med_2021_5965061 |
| ESTHER : Balaei-Kahnamoei_2021_Evid.Based.Complement.Alternat.Med_2021_5965061 |
| PubMedSearch : Balaei-Kahnamoei_2021_Evid.Based.Complement.Alternat.Med_2021_5965061 |
| PubMedID: 34335830 |
| Title : Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study - Szwajgier_2019_Curr.Alzheimer.Res_16_963 |
| Author(s) : Szwajgier D , Baranowska-Wojcik E |
| Ref : Curr Alzheimer Res , 16 :963 , 2019 |
| Abstract : Szwajgier_2019_Curr.Alzheimer.Res_16_963 |
| ESTHER : Szwajgier_2019_Curr.Alzheimer.Res_16_963 |
| PubMedSearch : Szwajgier_2019_Curr.Alzheimer.Res_16_963 |
| PubMedID: 31660828 |
| Title : Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential - Loesche_2018_Bioorg.Chem_85_23 |
| Author(s) : Loesche A , Kowitsch A , Lucas SD , Al-Halabi Z , Sippl W , Al-Harrasi A , Csuk R |
| Ref : Bioorg Chem , 85 :23 , 2018 |
| Abstract : Loesche_2018_Bioorg.Chem_85_23 |
| ESTHER : Loesche_2018_Bioorg.Chem_85_23 |
| PubMedSearch : Loesche_2018_Bioorg.Chem_85_23 |
| PubMedID: 30599410 |
| Title : Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling - Jeon_2017_Neuropharmacol_113_100 |
| Author(s) : Jeon SJ , Lee HJ , Lee HE , Park SJ , Gwon Y , Kim H , Zhang J , Shin CY , Kim DH , Ryu JH |
| Ref : Neuropharmacology , 113 :100 , 2017 |
| Abstract : Jeon_2017_Neuropharmacol_113_100 |
| ESTHER : Jeon_2017_Neuropharmacol_113_100 |
| PubMedSearch : Jeon_2017_Neuropharmacol_113_100 |
| PubMedID: 27470063 |
| Title : Neurologically Potent Molecules from Crataegus oxyacantha\; Isolation, Anticholinesterase Inhibition, and Molecular Docking - Ali_2017_Front.Pharmacol_8_327 |
| Author(s) : Ali M , Muhammad S , Shah MR , Khan A , Rashid U , Farooq U , Ullah F , Sadiq A , Ayaz M , Ahmad M , Latif A |
| Ref : Front Pharmacol , 8 :327 , 2017 |
| Abstract : Ali_2017_Front.Pharmacol_8_327 |
| ESTHER : Ali_2017_Front.Pharmacol_8_327 |
| PubMedSearch : Ali_2017_Front.Pharmacol_8_327 |
| PubMedID: 28638340 |
| Title : Bioactive constituents of Salvia chrysophylla Stapf - Culhaoglu_2013_Nat.Prod.Res_27_438 |
| Author(s) : Culhaoglu B , Yapar G , Dirmenci T , Topcu G |
| Ref : Nat Prod Res , 27 :438 , 2013 |
| Abstract : Culhaoglu_2013_Nat.Prod.Res_27_438 |
| ESTHER : Culhaoglu_2013_Nat.Prod.Res_27_438 |
| PubMedSearch : Culhaoglu_2013_Nat.Prod.Res_27_438 |
| PubMedID: 23126495 |
| Title : Facile synthesis of three bidesmosidic oleanolic acid saponins with strong inhibitory activity on pancreatic lipase - Guo_2009_Carbohydr.Res_344_1167 |
| Author(s) : Guo T , Liu Q , Wang P , Zhang L , Zhang W , Li Y |
| Ref : Carbohydr Res , 344 :1167 , 2009 |
| Abstract : Guo_2009_Carbohydr.Res_344_1167 |
| ESTHER : Guo_2009_Carbohydr.Res_344_1167 |
| PubMedSearch : Guo_2009_Carbohydr.Res_344_1167 |
| PubMedID: 19463989 |
| Title : Oleanolic acid and related derivatives as medicinally important compounds - Sultana_2008_J.Enzyme.Inhib.Med.Chem_23_739 |
| Author(s) : Sultana N , Ata A |
| Ref : J Enzyme Inhib Med Chem , 23 :739 , 2008 |
| Abstract : Sultana_2008_J.Enzyme.Inhib.Med.Chem_23_739 |
| ESTHER : Sultana_2008_J.Enzyme.Inhib.Med.Chem_23_739 |
| PubMedSearch : Sultana_2008_J.Enzyme.Inhib.Med.Chem_23_739 |
| PubMedID: 18618318 |
| Title : Release of acetylcholine to raise insulin secretion in Wistar rats by oleanolic acid, one of the active principles contained in Cornus officinalis - Hsu_2006_Neurosci.Lett_404_112 |
| Author(s) : Hsu JH , Wu YC , Liu IM , Cheng JT |
| Ref : Neuroscience Letters , 404 :112 , 2006 |
| Abstract : Hsu_2006_Neurosci.Lett_404_112 |
| ESTHER : Hsu_2006_Neurosci.Lett_404_112 |
| PubMedSearch : Hsu_2006_Neurosci.Lett_404_112 |
| PubMedID: 16759806 |
| Title : Three new cholinesterase-inhibiting cis-clerodane diterpenoids from Otostegia limbata - Ahmad_2005_Chem.Pharm.Bull.(Tokyo)_53_378 |
| Author(s) : Ahmad VU , Khan A , Farooq U , Kousar F , Khan SS , Nawaz SA , Abbasi MA , Choudhary MI |
| Ref : Chem Pharm Bull (Tokyo) , 53 :378 , 2005 |
| Abstract : Ahmad_2005_Chem.Pharm.Bull.(Tokyo)_53_378 |
| ESTHER : Ahmad_2005_Chem.Pharm.Bull.(Tokyo)_53_378 |
| PubMedSearch : Ahmad_2005_Chem.Pharm.Bull.(Tokyo)_53_378 |
| PubMedID: 15802835 |
| Title : Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives - Ali_2002_Phytochemistry_60_295 |
| Author(s) : Ali MS , Jahangir M , Hussan SS , Choudhary MI |
| Ref : Phytochemistry , 60 :295 , 2002 |
| Abstract : Ali_2002_Phytochemistry_60_295 |
| ESTHER : Ali_2002_Phytochemistry_60_295 |
| PubMedSearch : Ali_2002_Phytochemistry_60_295 |
| PubMedID: 12031449 |