Nijmegen-1
the SL analogues (Nijmegen-1 MP3, MP16) are applied in the field as suicidal germination agent for parasitic weed (Orobanche) control
General
Type : Cotylimide,Strigolactone receptors ligand,Isoindole,Indole,Butenolide,Phthalimido
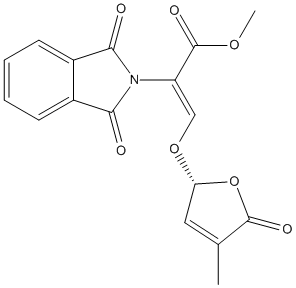
Chemical_Nomenclature : methyl (Z)-2-(1,3-dioxoisoindol-2-yl)-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxy]prop-2-enoate
Canonical SMILES : CC1=CC(OC1=O)OC=C(C(=O)OC)N2C(=O)C3=CC=CC=C3C2=O
InChI : InChI=1S\/C17H13NO7\/c1-9-7-13(25-16(9)21)24-8-12(17(22)23-2)18-14(19)10-5-3-4-6-11(10)15(18)20\/h3-8,13H,1-2H3\/b12-8-\/t13-\/m1\/s1
InChIKey : UBVNIMAEIBGUEL-LLBKUYECSA-N
Other name(s) : CHEMBL2270924,ZINC39007800,(2Z)-2-Phthalimidyl-3-[[(2R)-4-methyl-5-oxo-2,5-dihydrofuran]-2-yloxy]acrylic acid methyl ester
MW : 343.29
Formula : C17H13NO7
CAS_number :
PubChem : 10545295
UniChem : UBVNIMAEIBGUEL-LLBKUYECSA-N
IUPHAR :
Wikipedia :
Target
References (8)
| Title : Striga hermonthica Suicidal Germination Activity of Potent Strigolactone Analogs: Evaluation from Laboratory Bioassays to Field Trials - Jamil_2022_Plants.(Basel)_11__ |
| Author(s) : Jamil M , Wang JY , Yonli D , Ota T , Berqdar L , Traore H , Margueritte O , Zwanenburg B , Asami T , Al-Babili S |
| Ref : Plants (Basel) , 11 : , 2022 |
| Abstract : Jamil_2022_Plants.(Basel)_11__ |
| ESTHER : Jamil_2022_Plants.(Basel)_11__ |
| PubMedSearch : Jamil_2022_Plants.(Basel)_11__ |
| PubMedID: 35448773 |
| Title : Suicidal germination for parasitic weed control - Zwanenburg_2016_Pest.Manag.Sci_72_2016 |
| Author(s) : Zwanenburg B , Mwakaboko AS , Kannan C |
| Ref : Pest Manag Sci , 72 :2016 , 2016 |
| Abstract : Zwanenburg_2016_Pest.Manag.Sci_72_2016 |
| ESTHER : Zwanenburg_2016_Pest.Manag.Sci_72_2016 |
| PubMedSearch : Zwanenburg_2016_Pest.Manag.Sci_72_2016 |
| PubMedID: 26733056 |
| Title : Strigolactone Analogues with a D-Ring Modified at C-2 - Mwakaboko_2016_European.J.Org.Chem_2016_3495 |
| Author(s) : Mwakaboko AS , Zwanenburg B |
| Ref : European J Org Chem , 2016 :3495 , 2016 |
| Abstract : Mwakaboko_2016_European.J.Org.Chem_2016_3495 |
| ESTHER : Mwakaboko_2016_European.J.Org.Chem_2016_3495 |
| PubMedSearch : Mwakaboko_2016_European.J.Org.Chem_2016_3495 |
| PubMedID: 27840586 |
| Title : Structure and activity of strigolactones: new plant hormones with a rich future - Zwanenburg_2013_Mol.Plant_6_38 |
| Author(s) : Zwanenburg B , Pospisil T |
| Ref : Mol Plant , 6 :38 , 2013 |
| Abstract : Zwanenburg_2013_Mol.Plant_6_38 |
| ESTHER : Zwanenburg_2013_Mol.Plant_6_38 |
| PubMedSearch : Zwanenburg_2013_Mol.Plant_6_38 |
| PubMedID: 23204499 |
| Title : Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds - Zwanenburg_2011_Bioorg.Med.Chem_19_7394 |
| Author(s) : Zwanenburg B , Mwakaboko AS |
| Ref : Bioorganic & Medicinal Chemistry , 19 :7394 , 2011 |
| Abstract : Zwanenburg_2011_Bioorg.Med.Chem_19_7394 |
| ESTHER : Zwanenburg_2011_Bioorg.Med.Chem_19_7394 |
| PubMedSearch : Zwanenburg_2011_Bioorg.Med.Chem_19_7394 |
| PubMedID: 22082666 |
| Title : A small-molecule screen identifies new functions for the plant hormone strigolactone - Tsuchiya_2010_Nat.Chem.Biol_6_741 |
| Author(s) : Tsuchiya Y , Vidaurre D , Toh S , Hanada A , Nambara E , Kamiya Y , Yamaguchi S , McCourt P |
| Ref : Nat Chemical Biology , 6 :741 , 2010 |
| Abstract : Tsuchiya_2010_Nat.Chem.Biol_6_741 |
| ESTHER : Tsuchiya_2010_Nat.Chem.Biol_6_741 |
| PubMedSearch : Tsuchiya_2010_Nat.Chem.Biol_6_741 |
| PubMedID: 20818397 |
| Title : SAR studies of sesquiterpene lactones as Orobanche cumana seed germination stimulants - Galindo_2002_J.Agric.Food.Chem_50_1911 |
| Author(s) : Galindo JC , de Luque AP , Jorrin J , Macias FA |
| Ref : Journal of Agricultural and Food Chemistry , 50 :1911 , 2002 |
| Abstract : Galindo_2002_J.Agric.Food.Chem_50_1911 |
| ESTHER : Galindo_2002_J.Agric.Food.Chem_50_1911 |
| PubMedSearch : Galindo_2002_J.Agric.Food.Chem_50_1911 |
| PubMedID: 11902932 |
| Title : Dose-response of seeds of the parasitic weeds Striga and Orobanche toward the synthetic germination stimulants GR 24 and Nijmegen 1 - Wigchert_1999_J.Agric.Food.Chem_47_1705 |
| Author(s) : Wigchert SC , Kuiper E , Boelhouwer GJ , Nefkens GH , Verkleij JA , Zwanenburg B |
| Ref : Journal of Agricultural and Food Chemistry , 47 :1705 , 1999 |
| Abstract : Wigchert_1999_J.Agric.Food.Chem_47_1705 |
| ESTHER : Wigchert_1999_J.Agric.Food.Chem_47_1705 |
| PubMedSearch : Wigchert_1999_J.Agric.Food.Chem_47_1705 |
| PubMedID: 10564042 |