NF595
very similar to Bis7-tacrine
General
Type : Derivative of Tacrine,Alkyl linked bis-ligand,Sulfur Compound
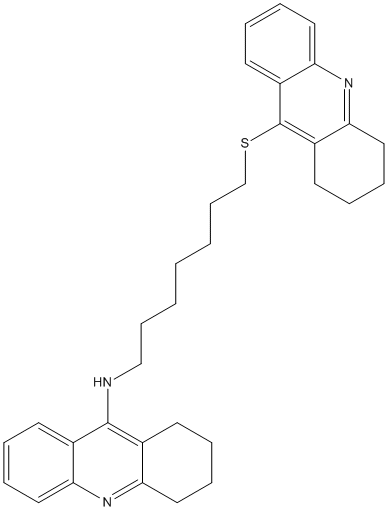
Chemical_Nomenclature : N-[8-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)octyl]-1,2,3,4-tetrahydroacridin-9-amine
Canonical SMILES : C1CCC2=NC3=CC=CC=C3C(=C2C1)NCCCCCCCCSC4=C5CCCCC5=NC6=CC=CC=C64
InChI : InChI=1S\/C34H41N3S\/c1(3-13-23-35-33-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)33)2-4-14-24-38-34-27-17-7-11-21-31(27)37-32-22-12-8-18-28(32)34\/h5,7,9,11,15,17,19,21H,1-4,6,8,10,12-14,16,18,20,22-24H2,(H,35,36)
InChIKey : COEYCPFFRLYNSC-UHFFFAOYSA-N
Other name(s) : CHEMBL129108,N8T,N-[8-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)octyl]-1,2,3,4-tetrahydroacridin-9-amine,Tacrine Dimer 4g,AC1O70TL,BDBM8971
MW : 523.77
Formula : C34H41N3S
CAS_number :
PubChem : 6540271
UniChem : COEYCPFFRLYNSC-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : NF595 ligand of proteins in family: ACHE
Stucture : 2CEK Conformational Flexibility in the Peripheral Site of Torpedo californica Acetylcholinesterase Revealed by the Complex Structure with a Bifunctional Inhibitor
Protein : torca-ACHE
References (1)
| Title : Conformational flexibility in the peripheral site of Torpedo californica acetylcholinesterase revealed by the complex structure with a bifunctional inhibitor - Colletier_2006_J.Am.Chem.Soc_128_4526 |
| Author(s) : Colletier JP , Sanson B , Nachon F , Gabellieri E , Fattorusso C , Campiani G , Weik M |
| Ref : Journal of the American Chemical Society , 128 :4526 , 2006 |
| Abstract : Colletier_2006_J.Am.Chem.Soc_128_4526 |
| ESTHER : Colletier_2006_J.Am.Chem.Soc_128_4526 |
| PubMedSearch : Colletier_2006_J.Am.Chem.Soc_128_4526 |
| PubMedID: 16594661 |
| Gene_locus related to this paper: torca-ACHE |