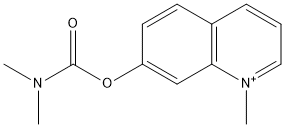
N-Methyl-7-dimethylcarbamoxyquinolinium
General
Type : Carbamate,Quinoline
Chemical_Nomenclature : N-Methyl-7-dimethylcarbamoxyquinolinium
Canonical SMILES : C[N+]1=CC=CC2=C1C=C(C=C2)OC(=O)N(C)C.[I-]
InChI : InChI=1S\/C13H15N2O2.HI\/c1-14(2)13(16)17-11-7-6-10-5-4-8-15(3)12(10)9-11\;\/h4-9H,1-3H3\;1H\/q+1\;\/p-1
InChIKey : YTLGJXQJZILJDB-UHFFFAOYSA-M
Other name(s) : M7C
MW : 231.27
Formula : C13H15N2O2+
CAS_number :
PubChem : 87845
UniChem : YTLGJXQJZILJDB-UHFFFAOYSA-M
IUPHAR :
Wikipedia :
Target
Families : N-Methyl-7-dimethylcarbamoxyquinolinium ligand of proteins in family: ACHE || BCHE
Stucture :
Protein :
References (6)
| Title : Peripheral site ligands accelerate inhibition of acetylcholinesterase by neutral organophosphates - Radic_2001_J.Appl.Toxicol_21 Suppl 1_S13 |
| Author(s) : Radic Z , Taylor P |
| Ref : J Appl Toxicol , 21 Suppl 1 :S13 , 2001 |
| Abstract : Radic_2001_J.Appl.Toxicol_21 Suppl 1_S13 |
| ESTHER : Radic_2001_J.Appl.Toxicol_21 Suppl 1_S13 |
| PubMedSearch : Radic_2001_J.Appl.Toxicol_21 Suppl 1_S13 |
| PubMedID: 11920914 |
| Title : Interaction of tetrahydroaminoacridine with acetylcholinesterase and butyrylcholinesterase - Berman_1992_Mol.Pharmacol_41_412 |
| Author(s) : Berman HA , Leonard K |
| Ref : Molecular Pharmacology , 41 :412 , 1992 |
| Abstract : Berman_1992_Mol.Pharmacol_41_412 |
| ESTHER : Berman_1992_Mol.Pharmacol_41_412 |
| PubMedSearch : Berman_1992_Mol.Pharmacol_41_412 |
| PubMedID: 1538717 |
| Title : Conformational plasticity of butyrylcholinesterase as revealed by high pressure experiments - Masson_1990_Biochim.Biophys.Acta_1041_223 |
| Author(s) : Masson P , Balny C |
| Ref : Biochimica & Biophysica Acta , 1041 :223 , 1990 |
| Abstract : Masson_1990_Biochim.Biophys.Acta_1041_223 |
| ESTHER : Masson_1990_Biochim.Biophys.Acta_1041_223 |
| PubMedSearch : Masson_1990_Biochim.Biophys.Acta_1041_223 |
| PubMedID: 2268667 |
| Title : Effects of high pressure on the single-turnover kinetics of the carbamylation of cholinesterase - Masson_1988_Biochim.Biophys.Acta_954_208 |
| Author(s) : Masson P , Balny C |
| Ref : Biochimica & Biophysica Acta , 954 :208 , 1988 |
| Abstract : Masson_1988_Biochim.Biophys.Acta_954_208 |
| ESTHER : Masson_1988_Biochim.Biophys.Acta_954_208 |
| PubMedSearch : Masson_1988_Biochim.Biophys.Acta_954_208 |
| PubMedID: 3365437 |
| Title : Site selectivity of fluorescent bisquaternary phenanthridinium ligands for acetylcholinesterase - Berman_1987_Mol.Pharmacol_31_610 |
| Author(s) : Berman HA , Decker MM , Nowak MW , Leonard KJ , McCauley M , Baker WM , Taylor P |
| Ref : Molecular Pharmacology , 31 :610 , 1987 |
| Abstract : Berman_1987_Mol.Pharmacol_31_610 |
| ESTHER : Berman_1987_Mol.Pharmacol_31_610 |
| PubMedSearch : Berman_1987_Mol.Pharmacol_31_610 |
| PubMedID: 3600605 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : Taylor_1975_Biochemistry_14_1989 |
| ESTHER : Taylor_1975_Biochemistry_14_1989 |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |