Mipafox
Direct-acting neuropathy target esterase (NTE) inhibitor. Amide analog of DFP. M44 CID 44608019 is the hydrolysed form found in crystal structure of organophosphate anhydrolase/prolidase 3L7G
General
Type : Organophosphate
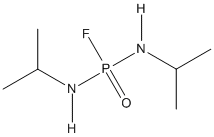
Chemical_Nomenclature : N-[fluoro-(propan-2-ylamino)phosphoryl]propan-2-amine
Canonical SMILES : CC(C)NP(=O)(NC(C)C)F
InChI : InChI=1S\/C6H16FN2OP\/c1-5(2)8-11(7,10)9-6(3)4\/h5-6H,1-4H3,(H2,8,9,10)
InChIKey : UOSHUBFBCPGQAY-UHFFFAOYSA-N
Other name(s) : N,N'-Bis(1-methylethyl)phosphorodiamidic fluoride,N,N'-diisopropylphosphorodiamidic fluoride,bis(isopropylamino)fluorophosphine oxide,Isopestox,Pestox XV,MIP
MW : 182.18
Formula : C6H16FN2OP
CAS_number : 371-86-8
PubChem : 9738
UniChem : UOSHUBFBCPGQAY-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (11)
| Title : Resolving pathways of interaction of mipafox and a sarin analog with human acetylcholinesterase by kinetics, mass spectrometry and molecular modeling approaches - Mangas_2016_Arch.Toxicol_90_603 |
| Author(s) : Mangas I , Taylor P , Vilanova E , Estevez J , Franca TCC , Komives E , Radic Z |
| Ref : Archives of Toxicology , 90 :603 , 2016 |
| Abstract : Mangas_2016_Arch.Toxicol_90_603 |
| ESTHER : Mangas_2016_Arch.Toxicol_90_603 |
| PubMedSearch : Mangas_2016_Arch.Toxicol_90_603 |
| PubMedID: 25743373 |
| Title : Further studies toward a mouse model for biochemical assessment of neuropathic potential of organophosphorus compounds - Makhaeva_2014_J.Appl.Toxicol_34_1426 |
| Author(s) : Makhaeva GF , Rudakova EV , Hein ND , Serebryakova OG , Kovaleva NV , Boltneva NP , Fink JK , Richardson RJ |
| Ref : J Appl Toxicol , 34 :1426 , 2014 |
| Abstract : Makhaeva_2014_J.Appl.Toxicol_34_1426 |
| ESTHER : Makhaeva_2014_J.Appl.Toxicol_34_1426 |
| PubMedSearch : Makhaeva_2014_J.Appl.Toxicol_34_1426 |
| PubMedID: 24395470 |
| Title : Organophosphate inhibition of human heart muscle cholinesterase isoenzymes - Chemnitius_1999_Chem.Biol.Interact_119-120_183 |
| Author(s) : Chemnitius JM , Sadowski R , Winkel H , Zech R |
| Ref : Chemico-Biological Interactions , 119-120 :183 , 1999 |
| Abstract : Chemnitius_1999_Chem.Biol.Interact_119-120_183 |
| ESTHER : Chemnitius_1999_Chem.Biol.Interact_119-120_183 |
| PubMedSearch : Chemnitius_1999_Chem.Biol.Interact_119-120_183 |
| PubMedID: 10421452 |
| Title : A comparison of the electrophysiological effects of two organophosphates, mipafox and ecothiopate, on mouse limb muscles - de Blaquiere_1998_Toxicol.Appl.Pharmacol_150_350 |
| Author(s) : de Blaquiere GE , Williams FM , Blain PG , Kelly SS |
| Ref : Toxicol Appl Pharmacol , 150 :350 , 1998 |
| Abstract : de Blaquiere_1998_Toxicol.Appl.Pharmacol_150_350 |
| ESTHER : de Blaquiere_1998_Toxicol.Appl.Pharmacol_150_350 |
| PubMedSearch : de Blaquiere_1998_Toxicol.Appl.Pharmacol_150_350 |
| PubMedID: 9653066 |
| Title : Inhibition of carboxylesterases in SH-SY5Y human and NB41A3 mouse neuroblastoma cells by organophosphorus esters - Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| Author(s) : Ehrich M , Correll L |
| Ref : J Toxicol Environ Health , 53 :385 , 1998 |
| Abstract : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| ESTHER : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedSearch : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedID: 9515941 |
| Title : Acetylcholinesterase and neuropathy target esterase inhibitions in neuroblastoma cells to distinguish organophosphorus compounds causing acute and delayed neurotoxicity - Ehrich_1997_Fundam.Appl.Toxicol_38_55 |
| Author(s) : Ehrich M , Correll L , Veronesi B |
| Ref : Fundamental & Applied Toxicology , 38 :55 , 1997 |
| Abstract : Ehrich_1997_Fundam.Appl.Toxicol_38_55 |
| ESTHER : Ehrich_1997_Fundam.Appl.Toxicol_38_55 |
| PubMedSearch : Ehrich_1997_Fundam.Appl.Toxicol_38_55 |
| PubMedID: 9268605 |
| Title : Mipafox differential inhibition assay for heart muscle cholinesterases: substrate specificity and inhibition of three isoenzymes by physostigmine and quinidine - Chemnitius_1997_Gen.Pharmacol_28_567 |
| Author(s) : Chemnitius JM , Haselmeyer KH , Gonska BD , Kreuzer H , Zech R |
| Ref : General Pharmacology , 28 :567 , 1997 |
| Abstract : Chemnitius_1997_Gen.Pharmacol_28_567 |
| ESTHER : Chemnitius_1997_Gen.Pharmacol_28_567 |
| PubMedSearch : Chemnitius_1997_Gen.Pharmacol_28_567 |
| PubMedID: 9147026 |
| Title : The effects of multiple low doses of organophosphates on target enzymes in brain and diaphragm in the mouse - Williams_1997_Human.Exp.Toxicol_16_67 |
| Author(s) : Williams FM , Charlton C , de Blaquiere GE , Mutch E , Kelly SS , Blain PG |
| Ref : Human & Experimental Toxicology , 16 :67 , 1997 |
| Abstract : Williams_1997_Human.Exp.Toxicol_16_67 |
| ESTHER : Williams_1997_Human.Exp.Toxicol_16_67 |
| PubMedSearch : Williams_1997_Human.Exp.Toxicol_16_67 |
| PubMedID: 9051410 |
| Title : Comparison of the relative inhibition of acetylcholinesterase and neuropathy target esterase in rats and hens given cholinesterase inhibitors - Ehrich_1995_Fundam.Appl.Toxicol_24_94 |
| Author(s) : Ehrich M , Jortner BS , Padilla S |
| Ref : Fundamental & Applied Toxicology , 24 :94 , 1995 |
| Abstract : Ehrich_1995_Fundam.Appl.Toxicol_24_94 |
| ESTHER : Ehrich_1995_Fundam.Appl.Toxicol_24_94 |
| PubMedSearch : Ehrich_1995_Fundam.Appl.Toxicol_24_94 |
| PubMedID: 7713347 |
| Title : Cholinesterases of heart muscle. Characterization of multiple enzymes using kinetics of irreversible organophosphorus inhibition - Chemnitius_1992_Biochem.Pharmacol_43_823 |
| Author(s) : Chemnitius JM , Chemnitius GC , Haselmeyer KH , Kreuzer H , Zech R |
| Ref : Biochemical Pharmacology , 43 :823 , 1992 |
| Abstract : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| ESTHER : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| PubMedSearch : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| PubMedID: 1540236 |
| Title : Mechanisms involved in the development of tolerance to DFP toxicity - Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| Author(s) : Gupta RC , Patterson GT , Dettbarn WD |
| Ref : Fundamental & Applied Toxicology , 5 :S17 , 1985 |
| Abstract : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| ESTHER : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| PubMedSearch : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| PubMedID: 4092885 |