Metolcarb
Metolcarb is an insecticide for the control of rice leafhoppers, planthoppers, codling moth, citrus mealybug, onion thrips, fruit flies, bollworms and aphids
General
Type : Insecticide,Carbamate
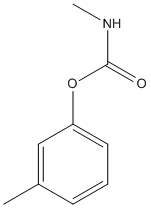
Chemical_Nomenclature : (3-methylphenyl) N-methylcarbamate
Canonical SMILES : CC1=CC(=CC=C1)OC(=O)NC
InChI : InChI=1S\/C9H11NO2\/c1-7-4-3-5-8(6-7)12-9(11)10-2\/h3-6H,1-2H3,(H,10,11)
InChIKey : VOEYXMAFNDNNED-UHFFFAOYSA-N
Other name(s) : M-Tolyl methylcarbamate,METOLCARB,Metacrate,Tsumacide,MTMC,3-Methylphenyl N-methylcarbamate
MW : 165.18
Formula : C9H11NO2
CAS_number : 1129-41-5
PubChem : 124489421
UniChem : VOEYXMAFNDNNED-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Metolcarb
Target
References (3)
| Title : Synthesis and insecticidal activity of novel carbamate derivatives as potential dual-binding site acetylcholinesterase inhibitors - Ma_2010_J.Agric.Food.Chem_58_12817 |
| Author(s) : Ma HJ , Xie RL , Zhao QF , Mei XD , Ning J |
| Ref : Journal of Agricultural and Food Chemistry , 58 :12817 , 2010 |
| Abstract : Ma_2010_J.Agric.Food.Chem_58_12817 |
| ESTHER : Ma_2010_J.Agric.Food.Chem_58_12817 |
| PubMedSearch : Ma_2010_J.Agric.Food.Chem_58_12817 |
| PubMedID: 21114293 |
| Title : Design of novel carbamate acetylcholinesterase inhibitors based on the multiple binding sites of acetylcholinesterase - Zhao_2008_J.Pestic.Sci_33_371 |
| Author(s) : Zhao Q , Yang G , Mei X , Yuan H , Ning J |
| Ref : Journal of Pesticide Science , 33 :371 , 2008 |
| Abstract : Zhao_2008_J.Pestic.Sci_33_371 |
| ESTHER : Zhao_2008_J.Pestic.Sci_33_371 |
| PubMedSearch : Zhao_2008_J.Pestic.Sci_33_371 |
| PubMedID: |
| Title : The Genotype and Heredity of Modified Acetylcholinesterase of the Green Rice Leafhopper (Nephotettix cincticeps Uhler) - Nomura_2000_Pestic.Biochem.Physiol_66_73 |
| Author(s) : Nomura M , Kato Y , Miyata T |
| Ref : Pesticide Biochemistry and Physiology , 66 :73 , 2000 |
| Abstract : Nomura_2000_Pestic.Biochem.Physiol_66_73 |
| ESTHER : Nomura_2000_Pestic.Biochem.Physiol_66_73 |
| PubMedSearch : Nomura_2000_Pestic.Biochem.Physiol_66_73 |
| PubMedID: |