Methylene-blue
An organic cation that is phenothiazin-5-ium substituted by dimethylamino groups at positions 3 and 7. The chloride salt is the histological dye 'methylene blue'. Solutions in water or alcohol have a deep blue color. Methylene blue is used as a bacteriologic stain and as an indicator (E-numbers for permitted antioxidant food additives are from E 300 to E 324). It inhibits guanylate cyclase, and has been used to treat cyanide poisoning and to lower levels of methemoglobin. Methylthioninium chloride (INN, or methylene blue, proposed trade name Rember) is an investigational drug being developed by the University of Aberdeen and TauRx Therapeutics that has been shown in early clinical trials to be an inhibitor of Tau protein aggregation. The drug is of potential interest for the treatment of patients with Alzheimer's disease
General
Type : Dye,Drug,Phenothiazine,anti-tau-aggregation,Multitarget,Sulfur Compound
Chemical_Nomenclature : [7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium
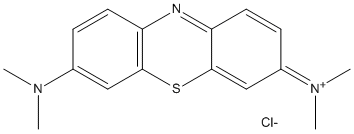
Canonical SMILES : CN(C)C1=CC2=C(C=C1)N=C3C=CC(=[N+](C)C)C=C3S2.[Cl-]
InChI : InChI=1S\/C16H18N3S.ClH\/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13\;\/h5-10H,1-4H3\;1H\/q+1\;\/p-1
InChIKey : RBTBFTRPCNLSDE-UHFFFAOYSA-N || CXKWCBBOMKCUKX-UHFFFAOYSA-M
Other name(s) : Methylene Blue cation,3,7-bis(dimethylamino)phenothiazin-5-ium,UNII-ZMZ79891ZH,CHEMBL191083,ZMZ79891ZH,CHEBI:43830,MBT,Methylene blue,Basic blue 9,Swiss Blue,Chromosmon,Methylthioninium chloride,Methylene Blue N
MW : 284.39
Formula : C16H18N3S
CAS_number : 61-73-4
UniChem : RBTBFTRPCNLSDE-UHFFFAOYSA-N || CXKWCBBOMKCUKX-UHFFFAOYSA-M
Wikipedia : Methylene_blue
Target
Families : Methylene-blue ligand of proteins in family
ACHE
BCHE
Protein :
torca-ACHE
References (14)
| Title : The interactions of azure B, a metabolite of methylene blue, with acetylcholinesterase and butyrylcholinesterase - Petzer_2014_Toxicol.Appl.Pharmacol_274_488 |
| Author(s) : Petzer A , Harvey BH , Petzer JP |
| Ref : Toxicol Appl Pharmacol , 274 :488 , 2014 |
| Abstract : Petzer_2014_Toxicol.Appl.Pharmacol_274_488 |
| ESTHER : Petzer_2014_Toxicol.Appl.Pharmacol_274_488 |
| PubMedSearch : Petzer_2014_Toxicol.Appl.Pharmacol_274_488 |
| PubMedID: 24161345 |
| Title : The specific interaction of the photosensitizer methylene blue with acetylcholinesterase provides a model system for studying the molecular consequences of photodynamic therapy - Silman_2013_Chem.Biol.Interact_203_63 |
| Author(s) : Silman I , Roth E , Paz A , Triquigneaux MM , Ehrenshaft M , Xu Y , Shnyrov VL , Sussman JL , Deterding LJ , Ashani Y , Mason RP , Weiner L |
| Ref : Chemico-Biological Interactions , 203 :63 , 2013 |
| Abstract : Silman_2013_Chem.Biol.Interact_203_63 |
| ESTHER : Silman_2013_Chem.Biol.Interact_203_63 |
| PubMedSearch : Silman_2013_Chem.Biol.Interact_203_63 |
| PubMedID: 23159732 |
| Title : Binding of methylene blue to a surface cleft inhibits the oligomerization and fibrillization of prion protein - Cavaliere_2013_Biochim.Biophys.Acta_1832_20 |
| Author(s) : Cavaliere P , Torrent J , Prigent S , Granata V , Pauwels K , Pastore A , Rezaei H , Zagari A |
| Ref : Biochimica & Biophysica Acta , 1832 :20 , 2013 |
| Abstract : Cavaliere_2013_Biochim.Biophys.Acta_1832_20 |
| ESTHER : Cavaliere_2013_Biochim.Biophys.Acta_1832_20 |
| PubMedSearch : Cavaliere_2013_Biochim.Biophys.Acta_1832_20 |
| PubMedID: 23022479 |
| Title : Determination of binding points of methylene blue and cationic phenoxazine dyes on human butyrylcholinesterase - Sezgin_2013_Arch.Biochem.Biophys_532_32 |
| Author(s) : Sezgin Z , Biberoglu K , Chupakhin V , Makhaeva GF , Tacal O |
| Ref : Archives of Biochemistry & Biophysics , 532 :32 , 2013 |
| Abstract : Sezgin_2013_Arch.Biochem.Biophys_532_32 |
| ESTHER : Sezgin_2013_Arch.Biochem.Biophys_532_32 |
| PubMedSearch : Sezgin_2013_Arch.Biochem.Biophys_532_32 |
| PubMedID: 23353050 |
| Title : Resistance of human butyrylcholinesterase to methylene blue-catalyzed photoinactivation\; mass spectrometry analysis of oxidation products - Tacal_2013_Photochem.Photobiol_89_336 |
| Author(s) : Tacal O , Li B , Lockridge O , Schopfer LM |
| Ref : Photochem Photobiol , 89 :336 , 2013 |
| Abstract : Tacal_2013_Photochem.Photobiol_89_336 |
| ESTHER : Tacal_2013_Photochem.Photobiol_89_336 |
| PubMedSearch : Tacal_2013_Photochem.Photobiol_89_336 |
| PubMedID: 23136924 |
| Title : Targeted oxidation of Torpedo californica acetylcholinesterase by singlet oxygen: identification of N-formylkynurenine tryptophan derivatives within the active-site gorge of its complex with the photosensitizer Methylene Blue - Triquigneaux_2012_Biochem.J_448_83 |
| Author(s) : Triquigneaux MM , Ehrenshaft M , Roth E , Silman I , Ashani Y , Mason RP , Weiner L , Deterding LJ |
| Ref : Biochemical Journal , 448 :83 , 2012 |
| Abstract : Triquigneaux_2012_Biochem.J_448_83 |
| ESTHER : Triquigneaux_2012_Biochem.J_448_83 |
| PubMedSearch : Triquigneaux_2012_Biochem.J_448_83 |
| PubMedID: 22888904 |
| Title : Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with Torpedo californica acetylcholinesterase - Paz_2012_Protein.Sci_21_1138 |
| Author(s) : Paz A , Roth E , Ashani Y , Xu Y , Shnyrov VL , Sussman JL , Silman I , Weiner L |
| Ref : Protein Science , 21 :1138 , 2012 |
| Abstract : Paz_2012_Protein.Sci_21_1138 |
| ESTHER : Paz_2012_Protein.Sci_21_1138 |
| PubMedSearch : Paz_2012_Protein.Sci_21_1138 |
| PubMedID: 22674800 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Comparative effects of cationic triarylmethane, phenoxazine and phenothiazine dyes on horse serum butyrylcholinesterase - Yucel_2008_Arch.Biochem.Biophys_478_201 |
| Author(s) : Yucel YY , Tacal O , Ozer I |
| Ref : Archives of Biochemistry & Biophysics , 478 :201 , 2008 |
| Abstract : Yucel_2008_Arch.Biochem.Biophys_478_201 |
| ESTHER : Yucel_2008_Arch.Biochem.Biophys_478_201 |
| PubMedSearch : Yucel_2008_Arch.Biochem.Biophys_478_201 |
| PubMedID: 18656440 |
| Title : Multi-site inhibition of human plasma cholinesterase by cationic phenoxazine and phenothiazine dyes - Kucukkilinc_2007_Arch.Biochem.Biophys_461_294 |
| Author(s) : Kucukkilinc TT , Ozer I |
| Ref : Archives of Biochemistry & Biophysics , 461 :294 , 2007 |
| Abstract : Kucukkilinc_2007_Arch.Biochem.Biophys_461_294 |
| ESTHER : Kucukkilinc_2007_Arch.Biochem.Biophys_461_294 |
| PubMedSearch : Kucukkilinc_2007_Arch.Biochem.Biophys_461_294 |
| PubMedID: 17428437 |
| Title : Methylene blue is a muscarinic antagonist in cardiac myocytes - Abi-Gerges_1997_Mol.Pharmacol_52_482 |
| Author(s) : Abi-Gerges N , Eschenhagen T , Hove-Madsen L , Fischmeister R , Mery PF |
| Ref : Molecular Pharmacology , 52 :482 , 1997 |
| Abstract : Abi-Gerges_1997_Mol.Pharmacol_52_482 |
| ESTHER : Abi-Gerges_1997_Mol.Pharmacol_52_482 |
| PubMedSearch : Abi-Gerges_1997_Mol.Pharmacol_52_482 |
| PubMedID: 9281611 |
| Title : The interaction between methylene blue and the cholinergic system - Pfaffendorf_1997_Br.J.Pharmacol_122_95 |
| Author(s) : Pfaffendorf M , Bruning TA , Batink HD , van Zwieten PA , Batnik HD |
| Ref : British Journal of Pharmacology , 122 :95 , 1997 |
| Abstract : Pfaffendorf_1997_Br.J.Pharmacol_122_95 |
| ESTHER : Pfaffendorf_1997_Br.J.Pharmacol_122_95 |
| PubMedSearch : Pfaffendorf_1997_Br.J.Pharmacol_122_95 |
| PubMedID: 9298533 |
| Title : Methaemoglobin production and reduction by methylene blue and the interaction of methylene blue with sodium nitrite in vivo - Marrs_1989_Hum.Toxicol_8_359 |
| Author(s) : Marrs TC , Bright JE , Inns RH |
| Ref : Hum Toxicol , 8 :359 , 1989 |
| Abstract : Marrs_1989_Hum.Toxicol_8_359 |
| ESTHER : Marrs_1989_Hum.Toxicol_8_359 |
| PubMedSearch : Marrs_1989_Hum.Toxicol_8_359 |
| PubMedID: 2807304 |
| Title : Photo-oxidation of crystalline chymotrypsin in the presence of methylene blue - |
| Author(s) : Weil L , James S , Buchert AR |
| Ref : Archives of Biochemistry & Biophysics , 46 :266 , 1953 |
| PubMedID: |
| Title : Methylene Blue as an Inhibitor of Acetylcholine-Esterase - Augustinsson_1950_Acta.Chem.Scand_4_536 |
| Author(s) : Augustinsson KB |
| Ref : Acta Chemica Scandinavica , 4 :536 , 1950 |
| Abstract : Augustinsson_1950_Acta.Chem.Scand_4_536 |
| ESTHER : Augustinsson_1950_Acta.Chem.Scand_4_536 |
| PubMedSearch : Augustinsson_1950_Acta.Chem.Scand_4_536 |
| PubMedID: |