Meropenem
General
Type : Antibiotic,Pyrrolidine,Carbamate,Sulfur Compound
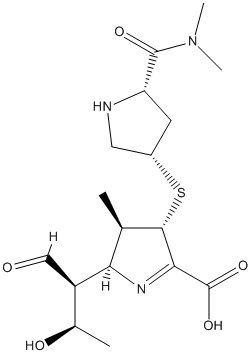
Chemical_Nomenclature : 2S,3R,4S)-4-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-2-[(2S,3R)-3-hydroxy-1-oxobutan-2-yl]-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid
Canonical SMILES : CC1C(C(=NC1C(C=O)C(C)O)C(=O)O)SC2CC(NC2)C(=O)N(C)C
InChI : InChI=1S\/C17H27N3O5S\/c1-8-13(11(7-21)9(2)22)19-14(17(24)25)15(8)26-10-5-12(18-6-10)16(23)20(3)4\/h7-13,15,18,22H,5-6H2,1-4H3,(H,24,25)\/t8-,9-,10+,11-,12+,13-,15+\/m1\/s1
InChIKey : UUIYVKJXUXGPKB-VGWSNGFZSA-N
Other name(s) : (2S,3R,4S)-4-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-2-[(2S,3R)-3-hydroxy-1-oxobutan-2-yl]-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid,Q27459765,MEPM,(2s,3r,4s)-4-{[(3s,5s)-5-(Dimethylcarbamoyl)pyrrolidin-3-Yl]sulfanyl}-2-[(1s,2r)-1-Formyl-2-Hydroxypropyl]-3-Methyl-3,4-Dihydro-2h-Pyrrole-5-Carboxylic Acid
MW : 385.5
Formula : C17H27N3O5S
CAS_number :
PubChem : 49866926
UniChem : UUIYVKJXUXGPKB-VGWSNGFZSA-N
IUPHAR :
Wikipedia :
Target
Families : Meropenem ligand of proteins in family: ACPH_Peptidase_S9
Stucture : 7QUN CryoEM structure of mammalian AAP in complex with Meropenem
Protein : pig-acph
References (2)
| Title : A carbapenem antibiotic inhibiting a mammalian serine protease: structure of the acylaminoacyl peptidase-meropenem complex - Kiss-Szeman_2022_Chem.Sci_13_14264 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Orgovan Z , Straner P , Jakli I , Schlosser G , Masiulis S , Harmat V , Menyhard DK , Perczel A |
| Ref : Chem Sci , 13 :14264 , 2022 |
| Abstract : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| ESTHER : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| PubMedSearch : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| PubMedID: 36545146 |
| Gene_locus related to this paper: pig-acph |
| Title : Characterization of inhibitory effect of carbapenem antibiotics on the deconjugation of valproic acid glucuronide - Masuo_2010_Drug.Metab.Dispos_38_1828 |
| Author(s) : Masuo Y , Ito K , Yamamoto T , Hisaka A , Honma M , Suzuki H |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1828 , 2010 |
| Abstract : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| ESTHER : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedSearch : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedID: 20581094 |