Meperidine
Meperidine is a narcotic analgesic that can be used for the relief of most types of moderate to severe pain, including postoperative pain and the pain of labor. Prolonged use may lead to dependence of the morphine type; withdrawal symptoms appear more rapidly than with morphine and are of shorter duration. Narcotic, sedative, analgesic, anesthetic. a combined mu and kappa-agonist
General
Type : Piperidine,Drug,Not A\/B H target,Opioid analgesic
Chemical_Nomenclature : 1-Methyl-4-phenyl-4-piperidinecarboxylic acid ethyl ester || ethyl 1-methyl-4-phenylpiperidine-4-carboxylate
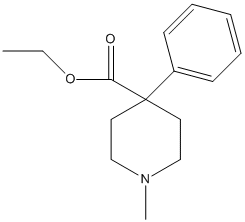
Canonical SMILES : CCOC(=O)C1(CCN(CC1)C)C2=CC=CC=C2
InChI : InChI=1S\/C15H21NO2\/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13\/h4-8H,3,9-12H2,1-2H3
InChIKey : XADCESSVHJOZHK-UHFFFAOYSA-N
Other name(s) : Demerol,Pethidine,Meperidine hydrochloride,Meperidine,Isonipecaine,Lidol,Pethanol,Piridosal,Algil,Alodan,Centralgin,Dispadol,Dolantin,Mialgin,Petidin Dolargan,Dolestine,Dolosal,Dolsin,Mefedina
MW : 247.34
Formula : C15H21NO2
CAS_number : 57-42-1
PubChem : 4058
UniChem : XADCESSVHJOZHK-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Pethidine
Target
Families : Meperidine ligand of proteins in family: BCHE || Carboxylesterase || Carb_B_Chordata
Stucture :
Protein : human-BCHE
References (6)
| Title : Binding and hydrolysis of meperidine by human liver carboxylesterase hCE-1 - Zhang_1999_J.Pharmacol.Exp.Ther_290_314 |
| Author(s) : Zhang J , Burnell JC , Dumaual N , Bosron WF |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 290 :314 , 1999 |
| Abstract : Zhang_1999_J.Pharmacol.Exp.Ther_290_314 |
| ESTHER : Zhang_1999_J.Pharmacol.Exp.Ther_290_314 |
| PubMedSearch : Zhang_1999_J.Pharmacol.Exp.Ther_290_314 |
| PubMedID: 10381793 |
| Title : Meperidine and alfentanil do not reduce the gain or maximum intensity of shivering - Ikeda_1998_Anesthesiology_88_858 |
| Author(s) : Ikeda T , Sessler DI , Tayefeh F , Negishi C , Turakhia M , Marder D , Bjorksten AR , Larson MD |
| Ref : Anesthesiology , 88 :858 , 1998 |
| Abstract : Ikeda_1998_Anesthesiology_88_858 |
| ESTHER : Ikeda_1998_Anesthesiology_88_858 |
| PubMedSearch : Ikeda_1998_Anesthesiology_88_858 |
| PubMedID: 9579492 |
| Title : Meperidine-induced generalized seizures with normal renal function - Marinella_1997_South.Med.J_90_556 |
| Author(s) : Marinella MA |
| Ref : South Med J , 90 :556 , 1997 |
| Abstract : Marinella_1997_South.Med.J_90_556 |
| ESTHER : Marinella_1997_South.Med.J_90_556 |
| PubMedSearch : Marinella_1997_South.Med.J_90_556 |
| PubMedID: 9160082 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Differential inhibition of plasma cholinesterase variants using the dibutyrate analogue of pancuronium bromide - Whittaker_1981_Hum.Hered_31_242 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Hum Hered , 31 :242 , 1981 |
| Abstract : Whittaker_1981_Hum.Hered_31_242 |
| ESTHER : Whittaker_1981_Hum.Hered_31_242 |
| PubMedSearch : Whittaker_1981_Hum.Hered_31_242 |
| PubMedID: 7287015 |