MF268
General
Type : Derivative of physostigmine-eserine,Carbamate,Morpholine,Indole
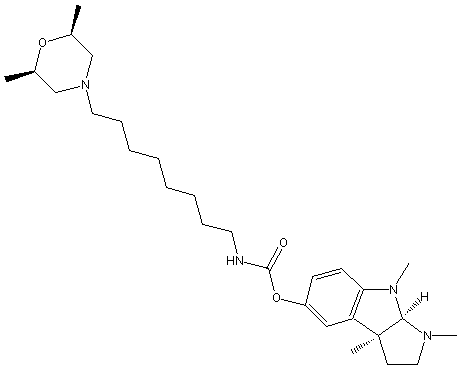
Chemical_Nomenclature : [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-[8-[(2S,6R)-2,6-dimethylmorpholin-4-yl]octyl]carbamate
Canonical SMILES : CC1CN(CC(O1)C)CCCCCCCCNC(=O)OC2=CC3=C(C=C2)N(C4C3(CCN4C)C)C
InChI : InChI=1S\/C28H46N4O3\/c1-21-19-32(20-22(2)34-21)16-11-9-7-6-8-10-15-29-27(33)35-23-12-13-25-24(18-23)28(3)14-17-30(4)26(28)31(25)5\/h12-13,18,21-22,26H,6-11,14-17,19-20H2,1-5H3,(H,29,33)\/t21-,22+,26-,28+\/m1\/s1
InChIKey : CJMJXTWMGXRFOM-FKTURHEWSA-N
Other name(s) : (3a S 8a R)-1,2,3,3a,8,8a-hexahydro 1,3a,8-trimethylpyrrolo[2,3-b]indol-5-ol[8-(cis2,6-dimethyl-morpholin-4 yl)octyl]-carbamate L-bitartrate hydrate,MF-268,MF 268,8-(2,6-Dimethylmorpholino)octylphysostigmine
MW : 486.69
Formula : C28H46N4O3
CAS_number : 154619-51-9
PubChem : 3074013
UniChem : CJMJXTWMGXRFOM-FKTURHEWSA-N
IUPHAR :
Wikipedia :
Target
Families : MF268 ligand of proteins in family: ACHE
Stucture : 1OCE Acetylcholinesterase + MF268
Protein : torca-ACHE
References (3)
| Title : Back Door Opening Implied by the Crystal Structure of a Carbamoylated Acetylcholinesterase - Bartolucci_1999_Biochemistry_38_5714 |
| Author(s) : Bartolucci C , Perola E , Cellai L , Brufani M , Lamba D |
| Ref : Biochemistry , 38 :5714 , 1999 |
| Abstract : Bartolucci_1999_Biochemistry_38_5714 |
| ESTHER : Bartolucci_1999_Biochemistry_38_5714 |
| PubMedSearch : Bartolucci_1999_Biochemistry_38_5714 |
| PubMedID: 10231521 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Long-lasting antiamnesic effect of a novel anticholinesterase inhibitor (MF268) - Braida_1998_Pharmacol.Biochem.Behav_59_897 |
| Author(s) : Braida D , Paladini E , Griffini P , Lamperti M , Colibretti L , Sala M |
| Ref : Pharmacol Biochem Behav , 59 :897 , 1998 |
| Abstract : Braida_1998_Pharmacol.Biochem.Behav_59_897 |
| ESTHER : Braida_1998_Pharmacol.Biochem.Behav_59_897 |
| PubMedSearch : Braida_1998_Pharmacol.Biochem.Behav_59_897 |
| PubMedID: 9586846 |
| Title : Long chain analogs of physostigmine as potential drugs for Alzheimer's disease: new insights into the mechanism of action in the inhibition of acetylcholinesterase. - Perola_1997_Biochim.Biophys.Acta_1343_41 |
| Author(s) : Perola E , Cellai L , Lamba D , Filocamo L , Brufani M |
| Ref : Biochimica & Biophysica Acta , 1343 :41 , 1997 |
| Abstract : Perola_1997_Biochim.Biophys.Acta_1343_41 |
| ESTHER : Perola_1997_Biochim.Biophys.Acta_1343_41 |
| PubMedSearch : Perola_1997_Biochim.Biophys.Acta_1343_41 |
| PubMedID: 9428657 |