LEI104
hDAGL-alpha LEI104: IC50=37+/-5 nM LEI104 is an alpha-ketoheterocycle that has been reported to inhibit fatty acid amide hydrolase (not A/B-hydrolase) Boger et al.
General
Type : Pyridine
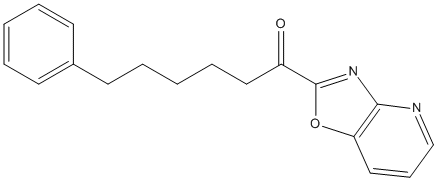
Chemical_Nomenclature : 1-([1,3]oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one
Canonical SMILES : C1=CC=C(C=C1)CCCCCC(=O)C2=NC3=C(O2)C=CC=N3
InChI : InChI=1S\/C18H18N2O2\/c21-15(18-20-17-16(22-18)12-7-13-19-17)11-6-2-5-10-14-8-3-1-4-9-14\/h1,3-4,7-9,12-13H,2,5-6,10-11H2
InChIKey : VPZHQLPAKFVGKX-UHFFFAOYSA-N
Other name(s) : PHOP,1-Oxazolo[4,5-b]pyridin-2-yl-6-phenyl-hexan-1-one,CHEMBL175854,1-([1,3]oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one,ZINC13611906,LEI-104,SCHEMBL3964367
MW : 294.3
Formula : C18H18N2O2
CAS_number : 288862-83-9
PubChem : 10085812
UniChem : VPZHQLPAKFVGKX-UHFFFAOYSA-N
Target
Families : LEI104 ligand of proteins in family
Lipase_3
Protein :
human-DAGLA
References (7)
| Title : Development of a Multiplexed Activity-Based Protein Profiling Assay to Evaluate Activity of Endocannabinoid Hydrolase Inhibitors - Janssen_2018_ACS.Chem.Biol_13_2406 |
| Author(s) : Janssen APA , van der Vliet D , Bakker AT , Jiang M , Grimm SH , Campiani G , Butini S , van der Stelt M |
| Ref : ACS Chemical Biology , 13 :2406 , 2018 |
| Abstract : Janssen_2018_ACS.Chem.Biol_13_2406 |
| ESTHER : Janssen_2018_ACS.Chem.Biol_13_2406 |
| PubMedSearch : Janssen_2018_ACS.Chem.Biol_13_2406 |
| PubMedID: 30199617 |
| Title : Highly Selective, Reversible Inhibitor Identified by Comparative Chemoproteomics Modulates Diacylglycerol Lipase Activity in Neurons - Baggelaar_2015_J.Am.Chem.Soc_137_8851 |
| Author(s) : Baggelaar MP , Chameau PJ , Kantae V , Hummel J , Hsu KL , Janssen F , van der Wel T , Soethoudt M , Deng H , den Dulk H , Allara M , Florea BI , Di Marzo V , Wadman WJ , Kruse CG , Overkleeft HS , Hankemeier T , Werkman TR , Cravatt BF , van der Stelt M |
| Ref : Journal of the American Chemical Society , 137 :8851 , 2015 |
| Abstract : Baggelaar_2015_J.Am.Chem.Soc_137_8851 |
| ESTHER : Baggelaar_2015_J.Am.Chem.Soc_137_8851 |
| PubMedSearch : Baggelaar_2015_J.Am.Chem.Soc_137_8851 |
| PubMedID: 26083464 |
| Gene_locus related to this paper: human-DAGLA , human-DAGLB |
| Title : Development of an activity-based probe and in silico design reveal highly selective inhibitors for diacylglycerol lipase-alpha in brain - |
| Author(s) : Baggelaar MP , Janssen FJ , van Esbroeck AC , den Dulk H , Allara M , Hoogendoorn S , McGuire R , Florea BI , Meeuwenoord N , van den Elst H , van der Marel GA , Brouwer J , Di Marzo V , Overkleeft HS , van der Stelt M |
| Ref : Angew Chem Int Ed Engl , 52 :12081 , 2013 |
| PubMedID: 24173880 |
| Gene_locus related to this paper: human-DAGLA |
| Title : High-performance liquid chromatographic assay with fluorescence detection for the evaluation of inhibitors against fatty acid amide hydrolase - Forster_2009_Anal.Bioanal.Chem_394_1679 |
| Author(s) : Forster L , Schulze Elfringhoff A , Lehr M |
| Ref : Anal Bioanal Chem , 394 :1679 , 2009 |
| Abstract : Forster_2009_Anal.Bioanal.Chem_394_1679 |
| ESTHER : Forster_2009_Anal.Bioanal.Chem_394_1679 |
| PubMedSearch : Forster_2009_Anal.Bioanal.Chem_394_1679 |
| PubMedID: 19484225 |
| Title : Exploration of a fundamental substituent effect of alpha-ketoheterocycle enzyme inhibitors: Potent and selective inhibitors of fatty acid amide hydrolase - DeMartino_2008_Bioorg.Med.Chem.Lett_18_5842 |
| Author(s) : DeMartino JK , Garfunkle J , Hochstatter DG , Cravatt BF , Boger DL |
| Ref : Bioorganic & Medicinal Chemistry Lett , 18 :5842 , 2008 |
| Abstract : DeMartino_2008_Bioorg.Med.Chem.Lett_18_5842 |
| ESTHER : DeMartino_2008_Bioorg.Med.Chem.Lett_18_5842 |
| PubMedSearch : DeMartino_2008_Bioorg.Med.Chem.Lett_18_5842 |
| PubMedID: 18639454 |
| Title : Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity - Lichtman_2004_J.Pharmacol.Exp.Ther_311_441 |
| Author(s) : Lichtman AH , Leung D , Shelton CC , Saghatelian A , Hardouin C , Boger DL , Cravatt BF |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 311 :441 , 2004 |
| Abstract : Lichtman_2004_J.Pharmacol.Exp.Ther_311_441 |
| ESTHER : Lichtman_2004_J.Pharmacol.Exp.Ther_311_441 |
| PubMedSearch : Lichtman_2004_J.Pharmacol.Exp.Ther_311_441 |
| PubMedID: 15229230 |
| Title : Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide - Boger_2000_Proc.Natl.Acad.Sci.U.S.A_97_5044 |
| Author(s) : Boger DL , Sato H , Lerner AE , Hedrick MP , Fecik RA , Miyauchi H , Wilkie GD , Austin BJ , Patricelli MP , Cravatt BF |
| Ref : Proc Natl Acad Sci U S A , 97 :5044 , 2000 |
| Abstract : Boger_2000_Proc.Natl.Acad.Sci.U.S.A_97_5044 |
| ESTHER : Boger_2000_Proc.Natl.Acad.Sci.U.S.A_97_5044 |
| PubMedSearch : Boger_2000_Proc.Natl.Acad.Sci.U.S.A_97_5044 |
| PubMedID: 10805767 |