LASSBio-767
derived from the natural piperidine alkaloid (-)-spectaline, obtained from the flowers of Senna spectabilis (Fabaceae)
General
Type : Natural,Derivative of Spectaline,Piperidine
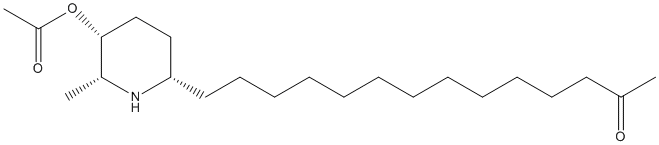
Chemical_Nomenclature : [(2R,3R,6S)-2-methyl-6-(13-oxotetradecyl)piperidin-3-yl] acetate
Canonical SMILES : CC1C(CCC(N1)CCCCCCCCCCCCC(=O)C)OC(=O)C
InChI : InChI=1S\/C22H41NO3\/c1-18(24)14-12-10-8-6-4-5-7-9-11-13-15-21-16-17-22(19(2)23-21)26-20(3)25\/h19,21-23H,4-17H2,1-3H3\/t19-,21+,22-\/m1\/s1
InChIKey : LRKWDKCSHJWJST-BAGYTPMASA-N
Other name(s) : (-)-3-O-acetyl-spectaline,CHEMBL251644,D08BPE,(?)-3-O-Acetylspectaline
MW : 367.57
Formula : C22H41NO3
CAS_number :
PubChem : 10474155
UniChem : LRKWDKCSHJWJST-BAGYTPMASA-N
IUPHAR :
Wikipedia :
Target
References (5)
| Title : CNS-selective noncompetitive cholinesterase inhibitors derived from the natural piperidine alkaloid (-)-spectaline - Castro_2008_Eur.J.Pharmacol_580_339 |
| Author(s) : Castro NG , Costa RS , Pimentel LS , Danuello A , Romeiro NC , Viegas C, Jr. , Barreiro EJ , Fraga CA , Bolzani VS , Rocha MS |
| Ref : European Journal of Pharmacology , 580 :339 , 2008 |
| Abstract : Castro_2008_Eur.J.Pharmacol_580_339 |
| ESTHER : Castro_2008_Eur.J.Pharmacol_580_339 |
| PubMedSearch : Castro_2008_Eur.J.Pharmacol_580_339 |
| PubMedID: 18096154 |
| Title : New selective acetylcholinesterase inhibitors designed from natural piperidine alkaloids - Viegas_2005_Bioorg.Med.Chem_13_4184 |
| Author(s) : Viegas C, Jr. , Bolzani VS , Pimentel LS , Castro NG , Cabral RF , Costa RS , Floyd C , Rocha MS , Young MC , Barreiro EJ , Fraga CA |
| Ref : Bioorganic & Medicinal Chemistry , 13 :4184 , 2005 |
| Abstract : Viegas_2005_Bioorg.Med.Chem_13_4184 |
| ESTHER : Viegas_2005_Bioorg.Med.Chem_13_4184 |
| PubMedSearch : Viegas_2005_Bioorg.Med.Chem_13_4184 |
| PubMedID: 15878668 |
| Title : New anti-Alzheimer drugs from biodiversity: the role of the natural acetylcholinesterase inhibitors - Viegas_2005_Mini.Rev.Med.Chem_5_915 |
| Author(s) : Viegas C, Jr. , Bolzani Vda S , Barreiro EJ , Fraga CA |
| Ref : Mini Rev Med Chem , 5 :915 , 2005 |
| Abstract : Viegas_2005_Mini.Rev.Med.Chem_5_915 |
| ESTHER : Viegas_2005_Mini.Rev.Med.Chem_5_915 |
| PubMedSearch : Viegas_2005_Mini.Rev.Med.Chem_5_915 |
| PubMedID: 16250834 |
| Title : Further bioactive piperidine alkaloids from the flowers and green fruits of Cassia spectabilis - Viegas_2004_J.Nat.Prod_67_908 |
| Author(s) : Viegas C, Jr. , da SBV , Furlan M , Barreiro EJ , Young MC , Tomazela D , Eberlin MN |
| Ref : Journal of Natural Products , 67 :908 , 2004 |
| Abstract : Viegas_2004_J.Nat.Prod_67_908 |
| ESTHER : Viegas_2004_J.Nat.Prod_67_908 |
| PubMedSearch : Viegas_2004_J.Nat.Prod_67_908 |
| PubMedID: 15165164 |