JW651
JW651 is a potent selective inhibitor of MAGL. JW651 inhibited mouse MAGL with an IC50 ~38 nM
General
Type : Piperazine,Halo ketone,Carbamate,Trifluoro
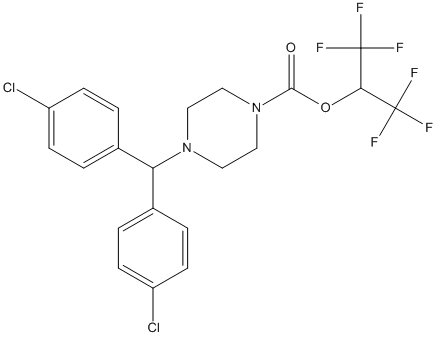
Chemical_Nomenclature : 1,1,1,3,3,3-Hexafluoropropan-2-yl 4-(bis(4-chlorophenyl)methyl)piperazine-1-carboxylate
Canonical SMILES : C1CN(CCN1C(C2=CC=C(C=C2)Cl)C3=CC=C(C=C3)Cl)C(=O)OC(C(F)(F)F)C(F)(F)F
InChI : InChI=1S\/C21H18Cl2F6N2O2\/c22-15-5-1-13(2-6-15)17(14-3-7-16(23)8-4-14)30-9-11-31(12-10-30)19(32)33-18(20(24,25)26)21(27,28)29\/h1-8,17-18H,9-12H2
InChIKey : UPIHQFKOCOKGEO-UHFFFAOYSA-N
Other name(s) : JW 651,JW-651,sml0990,SCHEMBL15100884,BDBM179938,ZINC205109968,US9133148, 2b
MW : 515.07
Formula : C21H18Cl2F6N2O2
CAS_number :
PubChem : 71656315
UniChem : UPIHQFKOCOKGEO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : JW651 ligand of proteins in family: Monoglyceridelipase_lysophospholip || Plasmodium_subtelomeric_PST-A
Stucture :
Protein : human-MGLL || plafa-PF10.0379
References (3)
| Title : Inhibition of Monoacylglycerol Lipase Decreases Angiogenic Features of Endothelial Cells via Release of Tissue Inhibitor of Metalloproteinase-1 from Lung Cancer Cells - Wittig_2023_Cells_12_1757 |
| Author(s) : Wittig F , Henkel L , Pruser JL , Merkord J , Ramer R , Hinz B |
| Ref : Cells , 12 :1757 , 2023 |
| Abstract : Wittig_2023_Cells_12_1757 |
| ESTHER : Wittig_2023_Cells_12_1757 |
| PubMedSearch : Wittig_2023_Cells_12_1757 |
| PubMedID: 37443791 |
| Gene_locus related to this paper: human-MGLL |
| Title : The Antimalarial Natural Product Salinipostin A Identifies Essential alpha\/beta Serine Hydrolases Involved in Lipid Metabolism in P. falciparum Parasites - Yoo_2020_Cell.Chem.Biol_27_143 |
| Author(s) : Yoo E , Schulze CJ , Stokes BH , Onguka O , Yeo T , Mok S , Gnadig NF , Zhou Y , Kurita K , Foe IT , Terrell SM , Boucher MJ , Cieplak P , Kumpornsin K , Lee MCS , Linington RG , Long JZ , Uhlemann AC , Weerapana E , Fidock DA , Bogyo M |
| Ref : Cell Chemical Biology , 27 :143 , 2020 |
| Abstract : Yoo_2020_Cell.Chem.Biol_27_143 |
| ESTHER : Yoo_2020_Cell.Chem.Biol_27_143 |
| PubMedSearch : Yoo_2020_Cell.Chem.Biol_27_143 |
| PubMedID: 31978322 |
| Gene_locus related to this paper: plaf7-q8ii19 , plafa-a0a143zya4 , plaf7-q8iik5 , plafa-MAL8P1.38 , plafa-PF07.0040 , plafa-PF10.0020 , plafa-PF10.0379 , plafa-PF13.0153 |
| Title : Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition - Chang_2013_ACS.Chem.Biol_8_1590 |
| Author(s) : Chang JW , Cognetta AB, 3rd , Niphakis MJ , Cravatt BF |
| Ref : ACS Chemical Biology , 8 :1590 , 2013 |
| Abstract : Chang_2013_ACS.Chem.Biol_8_1590 |
| ESTHER : Chang_2013_ACS.Chem.Biol_8_1590 |
| PubMedSearch : Chang_2013_ACS.Chem.Biol_8_1590 |
| PubMedID: 23701408 |