Iso-OMPA
Inhibitor specific of BChE and used to differentiate BChE activity from AChE activity (inhibited by BW284C51) (Austin and Berry 1953). Before this publication distinction of AChE and BChE was difficult: Nu-1250, inhibitor of AChE and Ro-2-0683 or Nu-683 ( or diisopropylphosphorofluoridate (DFP) ( Hawkins & Mendel,1947) to which BChE appears to be about 100-200 times as sensitive as AChE, were used.This compound was given the abbreviation iso-OMPA (Aldridge, 1953; Austin & Berry, 1953). This abbreviation incorrectly suggests that the compound is an isomer of OMPA (DuBois, Doull & Coon, 1950);
General
Type : Organophosphate
Chemical_Nomenclature : N-[bis(propan-2-ylamino)phosphoryloxy-(propan-2-ylamino)phosphoryl]propan-2-amine
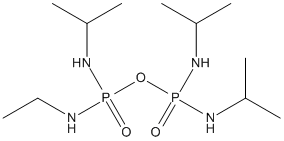
Canonical SMILES : CC(C)NP(=O)(NC(C)C)OP(=O)(NC(C)C)NC(C)C
InChI : InChI=1S\/C12H32N4O3P2\/c1-9(2)13-20(17,14-10(3)4)19-21(18,15-11(5)6)16-12(7)8\/h9-12H,1-8H3,(H2,13,14,17)(H2,15,16,18)
InChIKey : IOIMDJXKIMCMIG-UHFFFAOYSA-N
Other name(s) : Tetra monoisopropyl pyrophosphor tetramide,N,N'-diisopropyl pyrophosphore diamidic anhydre, T1505,DPDA,CHEMBL494887
MW : 342.4
Formula : C12H32N4O3P2
CAS_number : 513-00-8
PubChem : 5420
UniChem : IOIMDJXKIMCMIG-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (8)
| Title : Cholinesterase activity in human pulmonary arteries and veins - Walch_1997_Br.J.Pharmacol_121_986 |
| Author(s) : Walch L , Taisne C , Gascard JP , Nashashibi N , Brink C , Norel X |
| Ref : British Journal of Pharmacology , 121 :986 , 1997 |
| Abstract : Walch_1997_Br.J.Pharmacol_121_986 |
| ESTHER : Walch_1997_Br.J.Pharmacol_121_986 |
| PubMedSearch : Walch_1997_Br.J.Pharmacol_121_986 |
| PubMedID: 9222557 |
| Title : Purification and properties of monomeric (G1) forms of acetylcholinesterase secreted by Nippostrongylus brasiliensis - Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| Author(s) : Grigg ME , Tang L , Hussein AS , Selkirk ME |
| Ref : Molecular & Biochemical Parasitology , 90 :513 , 1997 |
| Abstract : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| ESTHER : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| PubMedSearch : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| PubMedID: 98135665 |
| Title : Site-directed mutagenesis of active site residues reveals plasticity of human butyrylcholinesterase in substrate and inhibitor interactions - Gnatt_1994_J.Neurochem_62_749 |
| Author(s) : Gnatt A , Loewenstein Y , Yaron A , Schwarz M , Soreq H |
| Ref : Journal of Neurochemistry , 62 :749 , 1994 |
| Abstract : Gnatt_1994_J.Neurochem_62_749 |
| ESTHER : Gnatt_1994_J.Neurochem_62_749 |
| PubMedSearch : Gnatt_1994_J.Neurochem_62_749 |
| PubMedID: 8294937 |
| Title : Degradation of acetylcholine in human airways: role of butyrylcholinesterase - Norel_1993_Br.J.Pharmacol_108_914 |
| Author(s) : Norel X , Angrisani M , Labat C , Gorenne I , Dulmet E , Rossi F , Brink C |
| Ref : British Journal of Pharmacology , 108 :914 , 1993 |
| Abstract : Norel_1993_Br.J.Pharmacol_108_914 |
| ESTHER : Norel_1993_Br.J.Pharmacol_108_914 |
| PubMedSearch : Norel_1993_Br.J.Pharmacol_108_914 |
| PubMedID: 8485630 |
| Title : Cholinesterases in rabbit serum - Simeon_1988_Gen.Pharmacol_19_849 |
| Author(s) : Simeon-Rudolf V , Reiner E , Skrinjaric-Spoljar M , Krauthacker B |
| Ref : General Pharmacology , 19 :849 , 1988 |
| Abstract : Simeon_1988_Gen.Pharmacol_19_849 |
| ESTHER : Simeon_1988_Gen.Pharmacol_19_849 |
| PubMedSearch : Simeon_1988_Gen.Pharmacol_19_849 |
| PubMedID: 3229626 |
| Title : Mechanisms involved in the development of tolerance to DFP toxicity - Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| Author(s) : Gupta RC , Patterson GT , Dettbarn WD |
| Ref : Fundamental & Applied Toxicology , 5 :S17 , 1985 |
| Abstract : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| ESTHER : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| PubMedSearch : Gupta_1985_Fundam.Appl.Toxicol_5_S17 |
| PubMedID: 4092885 |
| Title : Selective, near-total, irreversible inactivation of peripheral pseudocholinesterase and acetylcholinesterase in cats in vivo - |
| Author(s) : Koelle GB , Davis R , Diliberto EJJr , Koelle WA |
| Ref : Biochemical Pharmacology , 23 :175 , 1974 |
| PubMedID: 4360344 |