Iodoacetamide
Alkylating reagent commonly used to irreversibly label cysteines in biomolecules. Covalent modification by iodoacetamide at Cys-209 of Ag85C inactivates the enzyme
General
Type : Thiol-reactive
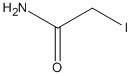
Chemical_Nomenclature : 2-iodoacetamide
Canonical SMILES : C(C(=O)N)I
InChI : InChI=1S\/C2H4INO\/c3-1-2(4)5\/h1H2,(H2,4,5)
InChIKey : PGLTVOMIXTUURA-UHFFFAOYSA-N
Other name(s) : 2-Iodoacetamide,Monoiodoacetamide,Surauto,Actamide, 2-iodo-
MW : 184.96
Formula : C2H4INO
CAS_number : 144-48-9
PubChem : 3727
UniChem : PGLTVOMIXTUURA-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Iodoacetamide ligand of proteins in family: A85-Mycolyl-transferase
Stucture : 4QDT Crystal structure of Antigen 85C co-crystallized with iodoacetamide
Protein : myctu-a85c
References (2)
| Title : Inactivation of the Mycobacterium tuberculosis antigen 85 complex by covalent, allosteric inhibitors - Favrot_2014_J.Biol.Chem_289_25031 |
| Author(s) : Favrot L , Lajiness DH , Ronning DR |
| Ref : Journal of Biological Chemistry , 289 :25031 , 2014 |
| Abstract : Favrot_2014_J.Biol.Chem_289_25031 |
| ESTHER : Favrot_2014_J.Biol.Chem_289_25031 |
| PubMedSearch : Favrot_2014_J.Biol.Chem_289_25031 |
| PubMedID: 25028518 |
| Gene_locus related to this paper: myctu-a85c |
| Title : Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen - Favrot_2013_Nat.Commun_4_2748 |
| Author(s) : Favrot L , Grzegorzewicz AE , Lajiness DH , Marvin RK , Boucau J , Isailovic D , Jackson M , Ronning DR |
| Ref : Nat Commun , 4 :2748 , 2013 |
| Abstract : Favrot_2013_Nat.Commun_4_2748 |
| ESTHER : Favrot_2013_Nat.Commun_4_2748 |
| PubMedSearch : Favrot_2013_Nat.Commun_4_2748 |
| PubMedID: 24193546 |
| Gene_locus related to this paper: myctu-a85c |