IDFPen
Isopropyl dodec-11-enylfluorophosphonate is an organophosphorus ester that antagonizes the central cannabinoid (CB1) receptor and inhibits FAAH with similar potencies (IC50s = 2 nM).1,2 At 30 mg/kg, this compound inhibits 99% of the brain neuropathy target esterase-lysophospholipase, which is attributed to causing delayed toxicity in mice || . Called IDFP in the title of the publication of Manthei et al. but in fact is IDFPen
General
Type : Organophosphate,Multitarget,FAAH inhibitor
Chemical_Nomenclature : 12-[fluoro(propan-2-yloxy)phosphoryl]dodec-1-ene
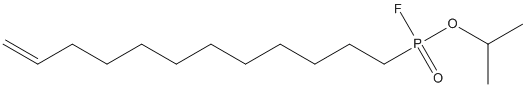
Canonical SMILES : CC(C)OP(=O)(CCCCCCCCCCC=C)F
InChI : InChI=1S\/C15H30FO2P\/c1-4-5-6-7-8-9-10-11-12-13-14-19(16,17)18-15(2)3\/h4,15H,1,5-14H2,2-3H3
InChIKey : HAGAHLUSUUTRJV-UHFFFAOYSA-N
Other name(s) : LP024089,ISOPROPYL DODEC-11-EN-1-YL(FLUORO)PHOSPHINATE,Isopropyl dodec-11-enylfluorophosphonate,Isopropyl-dodec-11-enylfluorophosphonate,Isopropyl dodec-11-en-1-yl(fluoro)phosphonate
MW : 292.36
Formula : C15H30FO2P
CAS_number : 623114-64-7
PubChem : 85883708
UniChem : HAGAHLUSUUTRJV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : IDFPen ligand of proteins in family: PC-sterol_acyltransferase
Stucture :
Protein : human-PLA2G15 || human-LCAT
References (1)
| Title : Structure and function of lysosomal phospholipase A2 and lecithin:cholesterol acyltransferase - Glukhova_2015_Nat.Commun_6_6250 |
| Author(s) : Glukhova A , Hinkovska-Galcheva V , Kelly R , Abe A , Shayman JA , Tesmer JJ |
| Ref : Nat Commun , 6 :6250 , 2015 |
| Abstract : Glukhova_2015_Nat.Commun_6_6250 |
| ESTHER : Glukhova_2015_Nat.Commun_6_6250 |
| PubMedSearch : Glukhova_2015_Nat.Commun_6_6250 |
| PubMedID: 25727495 |
| Gene_locus related to this paper: human-LCAT |