Hup-19
Compound Hup19 was grafted to a chromatographic support ECH-4B Sepharose to give an affinity gel Hupresin for efficient purification of BChE
General
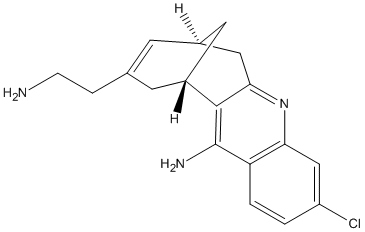
Type : Derivative of Huperzine
Chemical_Nomenclature : 3-Chloro-6,8-[2-(2-aminoethyl)-1-propene-1,3-diyl]-5,6,7,8-tetrahydroacridine-9-amine
Canonical SMILES : C1C2CC3=NC4=C(C=CC(=C4)Cl)C(=C3C1CC(=C2)CCN)N
InChI : InChI=1S\/C18H20ClN3\/c19-13-1-2-14-15(9-13)22-16-8-11-5-10(3-4-20)6-12(7-11)17(16)18(14)21\/h1-2,5,9,11-12H,3-4,6-8,20H2,(H2,21,22)
InChIKey : NHYOUQQOKSFBKN-UHFFFAOYSA-N
Other name(s) : SCHEMBL14665857,Hupresin,HUP-19,Huprine 19
MW : 313.82
Formula : C18H20ClN3
CAS_number :
PubChem : 53491574
UniChem : NHYOUQQOKSFBKN-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Hup-19 ligand of proteins in family: ACHE || BCHE
Stucture : 6EQQ Human butyrylcholinesterase in complex with huprine 19
Protein : human-BCHE
References (5)
| Title : Human butyrylcholinesterase in Cohn fraction IV-4 purified in a single chromatography step on Hupresin - Schopfer_2023_PLoS.One_18_e0280380 |
| Author(s) : Schopfer LM , David E , Hinrichs SH , Lockridge O |
| Ref : PLoS ONE , 18 :e0280380 , 2023 |
| Abstract : Schopfer_2023_PLoS.One_18_e0280380 |
| ESTHER : Schopfer_2023_PLoS.One_18_e0280380 |
| PubMedSearch : Schopfer_2023_PLoS.One_18_e0280380 |
| PubMedID: 36638134 |
| Title : Use of Hupresin To Capture Red Blood Cell Acetylcholinesterase for Detection of Soman Exposure - Onder_2018_Anal.Chem_90_974 |
| Author(s) : Onder S , Schopfer LM , Cashman JR , Tacal O , Johnson RC , Blake TA , Lockridge O |
| Ref : Analytical Chemistry , 90 :974 , 2018 |
| Abstract : Onder_2018_Anal.Chem_90_974 |
| ESTHER : Onder_2018_Anal.Chem_90_974 |
| PubMedSearch : Onder_2018_Anal.Chem_90_974 |
| PubMedID: 29172437 |
| Title : Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study - Rosenberry_2017_Molecules_22_ |
| Author(s) : Rosenberry TL , Brazzolotto X , Macdonald IR , Wandhammer M , Trovaslet-Leroy M , Darvesh S , Nachon F |
| Ref : Molecules , 22 : , 2017 |
| Abstract : Rosenberry_2017_Molecules_22_ |
| ESTHER : Rosenberry_2017_Molecules_22_ |
| PubMedSearch : Rosenberry_2017_Molecules_22_ |
| PubMedID: 29186056 |
| Gene_locus related to this paper: human-BCHE |
| Title : Human butyrylcholinesterase produced in insect cells: huprine-based affinity purification and crystal structure - Brazzolotto_2012_Febs.J_279_2905 |
| Author(s) : Brazzolotto X , Wandhammer M , Ronco C , Trovaslet M , Jean L , Lockridge O , Renard PY , Nachon F |
| Ref : Febs J , 279 :2905 , 2012 |
| Abstract : Brazzolotto_2012_Febs.J_279_2905 |
| ESTHER : Brazzolotto_2012_Febs.J_279_2905 |
| PubMedSearch : Brazzolotto_2012_Febs.J_279_2905 |
| PubMedID: 22726956 |
| Gene_locus related to this paper: human-BCHE |
| Title : New huprine derivatives functionalized at position 9 as highly potent acetylcholinesterase inhibitors - Ronco_2011_ChemMedChem_6_876 |
| Author(s) : Ronco C , Foucault R , Gillon E , Bohn P , Nachon F , Jean L , Renard PY |
| Ref : ChemMedChem , 6 :876 , 2011 |
| Abstract : Ronco_2011_ChemMedChem_6_876 |
| ESTHER : Ronco_2011_ChemMedChem_6_876 |
| PubMedSearch : Ronco_2011_ChemMedChem_6_876 |
| PubMedID: 21344648 |