Haloxon
anthelmintic, induce delayed neurotoxicity
General
Type : Organophosphate,Coumarin
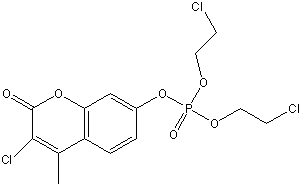
Chemical_Nomenclature : O,O-Di(2-chloroethyl)-O-(3-chloro-4-methylcoumarin-7-YL)phosphate
Canonical SMILES : CC1=C(C(=O)OC2=C1C=CC(=C2)OP(=O)(OCCCl)OCCCl)Cl
InChI : InChI=1S\/C14H14Cl3O6P\/c1-9-11-3-2-10(8-12(11)22-14(18)13(9)17)23-24(19,20-6-4-15)21-7-5-16\/h2-3,8H,4-7H2,1H3
InChIKey : KULDXINYXFTXMO-UHFFFAOYSA-N
Other name(s) : Eustidil,Galloxon,Galoxone,Helmirane,Helmiron,Helmirone,Loxon,Luxon
MW : 413.62
Formula : C15H16Cl3O5P
CAS_number : 321-55-1
PubChem : 9454
UniChem : KULDXINYXFTXMO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : Exploring the Active Sites of Cholinesterases by Inhibition with Bambuterol and Haloxon - Kovarik_2003_Croatica.Chem.Acta_76_63 |
| Author(s) : Kovarik Z , Bosac A , Sinko G , Latas T |
| Ref : Croatica Chemica Acta , 76 :63 , 2003 |
| Abstract : Kovarik_2003_Croatica.Chem.Acta_76_63 |
| ESTHER : Kovarik_2003_Croatica.Chem.Acta_76_63 |
| PubMedSearch : Kovarik_2003_Croatica.Chem.Acta_76_63 |
| PubMedID: |
| Gene_locus related to this paper: horse-BCHE |
| Title : The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases - Radic_1999_Chem.Biol.Interact_119-120_111 |
| Author(s) : Radic Z , Taylor P |
| Ref : Chemico-Biological Interactions , 119-120 :111 , 1999 |
| Abstract : Radic_1999_Chem.Biol.Interact_119-120_111 |
| ESTHER : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedSearch : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedID: 10421444 |
| Title : Role of the peripheral anionic site on acetylcholinesterase: inhibition by substrates and coumarin derivatives - Radic_1991_Mol.Pharmacol_39_98 |
| Author(s) : Radic Z , Reiner E , Taylor P |
| Ref : Molecular Pharmacology , 39 :98 , 1991 |
| Abstract : Radic_1991_Mol.Pharmacol_39_98 |
| ESTHER : Radic_1991_Mol.Pharmacol_39_98 |
| PubMedSearch : Radic_1991_Mol.Pharmacol_39_98 |
| PubMedID: 1987454 |